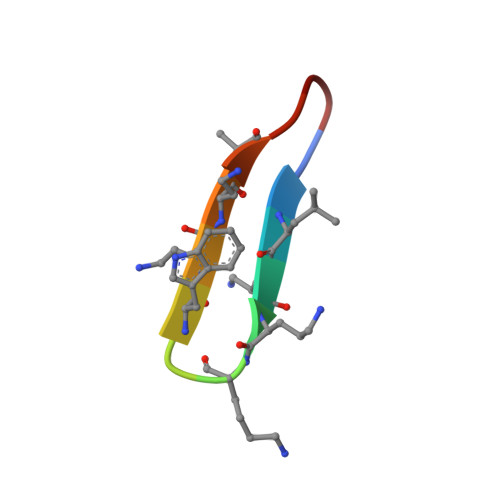
Structural studies of beta-hairpin peptidomimetic antibiotics that target LptD in Pseudomonas sp.
Schmidt, J., Patora-Komisarska, K., Moehle, K., Obrecht, D., Robinson, J.A.(2013) Bioorg Med Chem 21: 5806-5810
- PubMed: 23932450
- DOI: https://doi.org/10.1016/j.bmc.2013.07.013
- Primary Citation of Related Structures:
2M7I, 2M7J - PubMed Abstract:
We report structural studies in aqueous solution on backbone cyclic peptides that possess potent antimicrobial activity specifically against Pseudomonas sp. The peptides target the β-barrel outer membrane protein LptD, which plays an essential role in lipopolysaccharide transport to the outer membrane. The peptide L27-11 contains a 12-residue loop (T(1)W(2)L(3)K(4)K(5)R(6)R(7)W(8)K(9)K(10)A(11)K(12)) linked to a DPro-LPro template. Two related peptides were also studied, one with various Lys to ornithine or diaminobutyric acid substitutions as well as a DLys(6) (called LB-01), and another containing the same loop sequence, but linked to an LPro-DPro template (called LB-02). NMR studies and MD simulations show that L27-11 and LB-01 adopt β-hairpin structures in solution. In contrast, LB-02 is more flexible and importantly, adopts a wide variety of different backbone conformations, but not β-hairpin conformations. L27-11 and LB-01 show antimicrobial activity in the nanomolar range against Pseudomonas aeruginosa, whereas LB-02 is essentially inactive. Thus the β-hairpin structure of the peptide is important for antimicrobial activity. An alanine scan of L27-11 revealed that tryptophan side chains (W(2)/W(8)) displayed on opposite faces of the β-hairpin represent key groups contributing to antimicrobial activity.
- Chemistry Department, University of Zurich, Switzerland.
Organizational Affiliation: