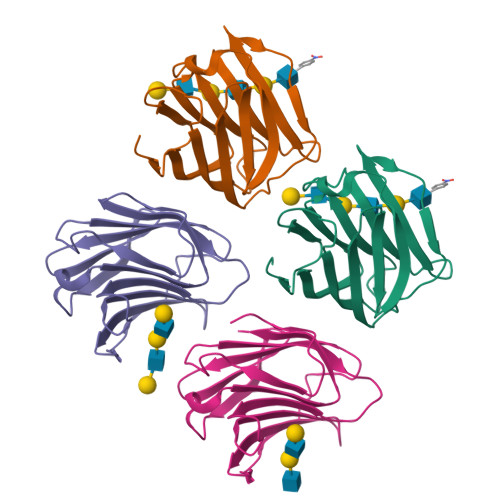
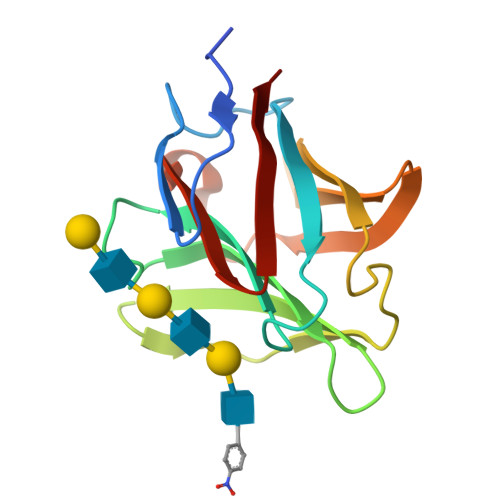
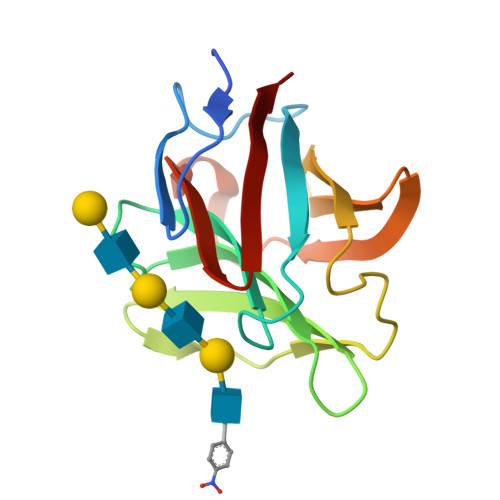
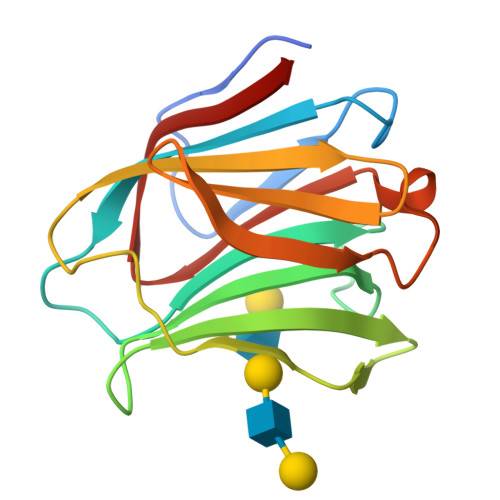
Structural analysis of the recognition mechanism of poly-N-acetyllactosamine by the human galectin-9 N-terminal carbohydrate recognition domain.
Nagae, M., Nishi, N., Murata, T., Usui, T., Nakamura, T., Wakatsuki, S., Kato, R.(2009) Glycobiology 19: 112-117
- PubMed: 18977853
- DOI: https://doi.org/10.1093/glycob/cwn121
- Primary Citation of Related Structures:
2ZHK, 2ZHL, 2ZHM, 2ZHN - PubMed Abstract:
Galectins are a family of beta-galactoside-specific lectins bearing a conserved carbohydrate recognition domain. Interactions between galectins and poly-N-acetyllactosamine sequences are critical in a variety of biological processes. Galectin-9, a member of the galectin family, has two carbohydrate recognition domains at both the N- and C-terminal regions. Here we report the crystal structure of the human galectin-9 N-terminal carbohydrate recognition domain in complex with N-acetyllactosamine dimers and trimers. These complex structures revealed that the galectin-9 N-terminal carbohydrate recognition domain can recognize internal N-acetyllactosamine units within poly-N-acetyllactosamine chains. Based on these complex structures, we propose two putative recognition modes for poly-N-acetyllactosamine binding by galectins.
Organizational Affiliation:
Structural Biology Research Center, Photon Factory, Institute of Materials Structure Science, High Energy Accelerator Research Organization (KEK), Tsukuba, Ibaraki, Japan.