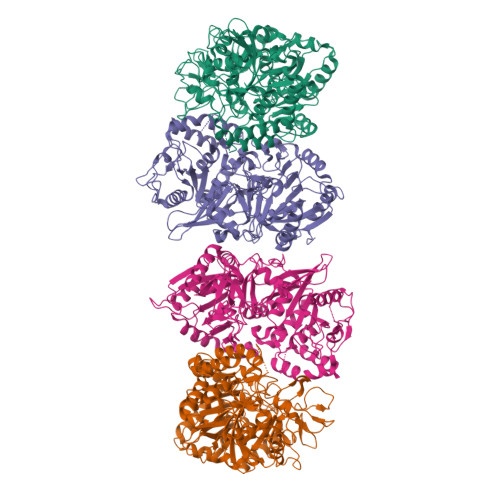
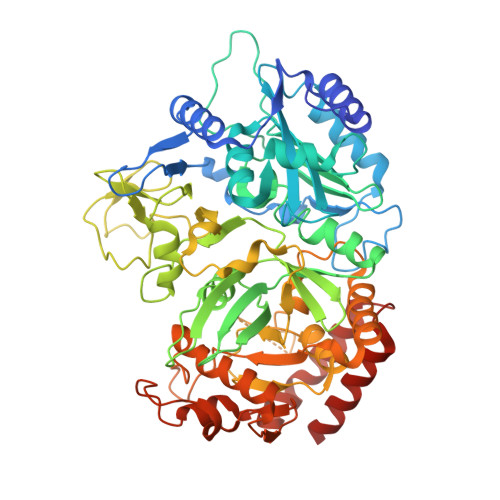
Structure of a GTP-dependent bacterial PEP-carboxykinase from Corynebacterium glutamicum.
Aich, S., Prasad, L., Delbaere, L.T.(2008) Int J Biochem Cell Biol 40: 1597-1603
- PubMed: 18234538
- DOI: https://doi.org/10.1016/j.biocel.2007.12.002
- Primary Citation of Related Structures:
2ZCI - PubMed Abstract:
GTP-dependent phosphoenolpyruvate carboxykinase (PCK) is the key enzyme that controls the blood glucose level during fasting in higher animals. Here we report the first substrate-free structure of a GTP-dependent phosphoenolpyruvate (PEP) carboxykinase from a bacterium, Corynebacterium glutamicum (CgPCK). The protein crystallizes in space group P2(1) with four molecules per asymmetric unit. The 2.3A resolution structure was solved by molecular replacement using the human cytosolic PCK (hcPCK) structure (PDB ID: 1KHF) as the starting model. The four molecules in the asymmetric unit pack as two dimers, and is an artifact of crystal packing. However, the P-loop and the guanine binding loop of the substrate-free CgPCK structure have different conformations from the other published GTP-specific PCK structures, which all have bound substrates and/or metal ions. It appears that a change in the P-loop and guanine binding loop conformation is necessary for substrate binding in GTP-specific PCKs, as opposed to overall domain movement in ATP-specific PCKs.
Organizational Affiliation:
Department of Biochemistry, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5E5, Canada.