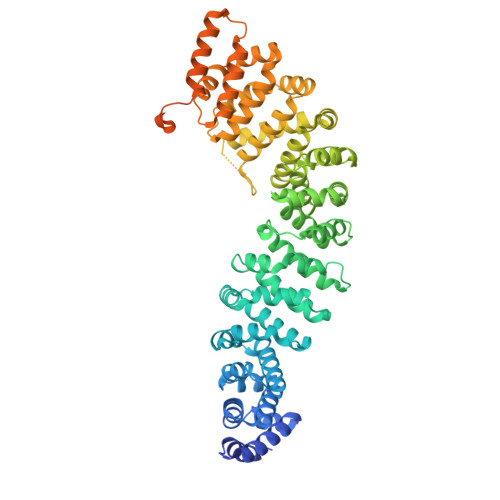
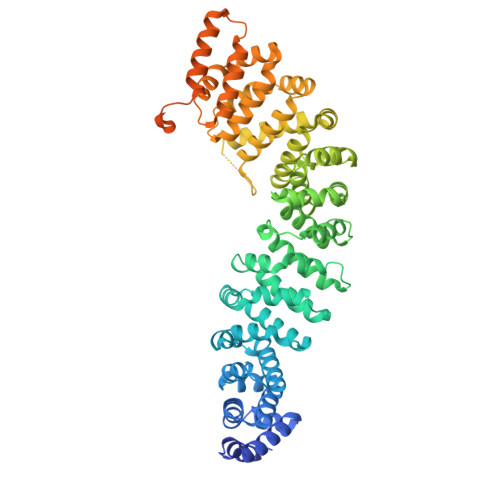
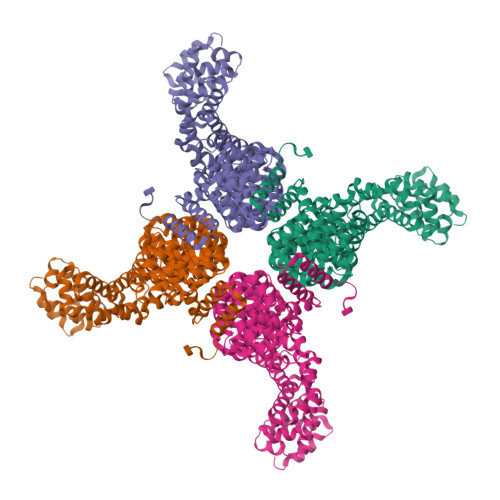
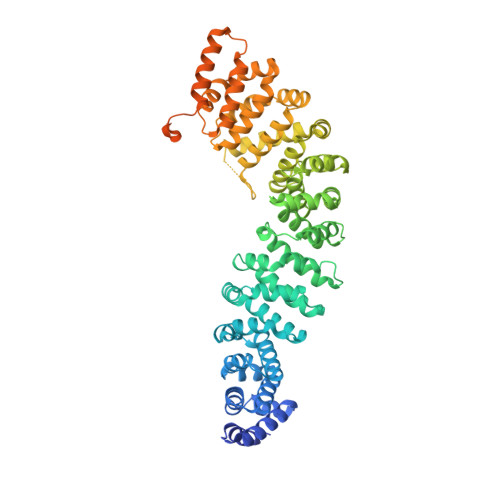
Crystal Structure of a Full-Length beta-Catenin
Xing, Y., Takemaru, K., Liu, J., Berndt, J.D., Zheng, J.J., Moon, R.T., Xu, W.(2008) Structure 16: 478-487
- PubMed: 18334222
- DOI: https://doi.org/10.1016/j.str.2007.12.021
- Primary Citation of Related Structures:
2Z6G, 2Z6H - PubMed Abstract:
beta-catenin plays essential roles in cell adhesion and Wnt signaling, while deregulation of beta-catenin is associated with multiple diseases including cancers. Here, we report the crystal structures of full-length zebrafish beta-catenin and a human beta-catenin fragment that contains both the armadillo repeat and the C-terminal domains. Our structures reveal that the N-terminal region of the C-terminal domain, a key component of the C-terminal transactivation domain, forms a long alpha helix that packs on the C-terminal end of the armadillo repeat domain, and thus forms part of the beta-catenin superhelical core. The existence of this helix redefines our view of interactions of beta-catenin with some of its critical partners, including ICAT and Chibby, which may form extensive interactions with this C-terminal domain alpha helix. Our crystallographic and NMR studies also suggest that the unstructured N-terminal and C-terminal tails interact with the ordered armadillo repeat domain in a dynamic and variable manner.
Organizational Affiliation:
Department of Biological Structure, University of Washington School of Medicine, Seattle, WA 98195, USA.