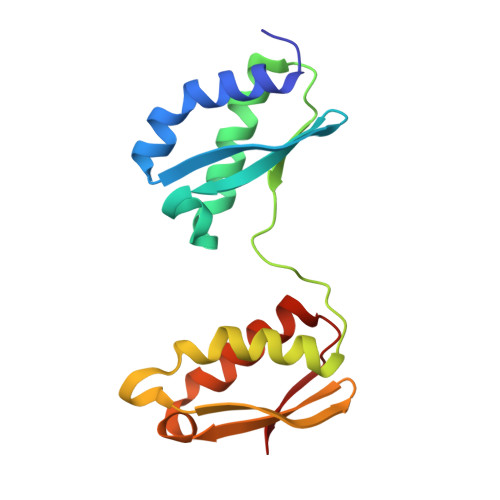
Structural Analysis of Arabidopsis CnfU Protein: An Iron-Sulfur Cluster Biosynthetic Scaffold in Chloroplasts.
Yabe, T., Yamashita, E., Kikuchi, A., Morimoto, K., Nakagawa, A., Tsukihara, T., Nakai, M.(2008) J Mol Biol
- PubMed: 18585737
- DOI: https://doi.org/10.1016/j.jmb.2008.05.072
- Primary Citation of Related Structures:
2Z51 - PubMed Abstract:
CnfU, a key iron-sulfur (Fe-S) cluster biosynthetic scaffold that is required for biogenesis of ferredoxin and photosystem I in chloroplasts, consists of two tandemly repeated domains in which only the N-terminal domain contains a conserved CXXC motif. We have determined the crystal structure of the metal-free dimer of AtCnfU-V from Arabidopsis thaliana at 1.35 A resolution. The N-terminal domains of the two monomers are linked together through two intermolecular disulfide bonds between the CXXC motifs. At the dimer interface, a total of four cysteine sulfur atoms provide a Fe-S cluster assembly site surrounded by uncharged but hydrophilic structurally mobile segments. The C-terminal domain of one monomer interacts with the N-terminal domain of the opposing monomer and thereby stabilizes dimer formation. Furthermore, Fe K-edge X-ray absorption spectroscopic analysis of the holo-CnfU dimer in solution suggests the presence of a typical [2Fe-2S]-type cluster coordinated by four thiolate ligands. Based on these data, a plausible model of the holo-AtCnfU-V dimer containing a surface-exposed [2Fe-2S] cluster assembled in the dimer interface was deduced. We propose that such a structural framework is important for CnfU to function as a Fe-S cluster biosynthetic scaffold.
Organizational Affiliation:
Laboratory of Regulation of Biological Reactions, Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita 565-0871, Japan.