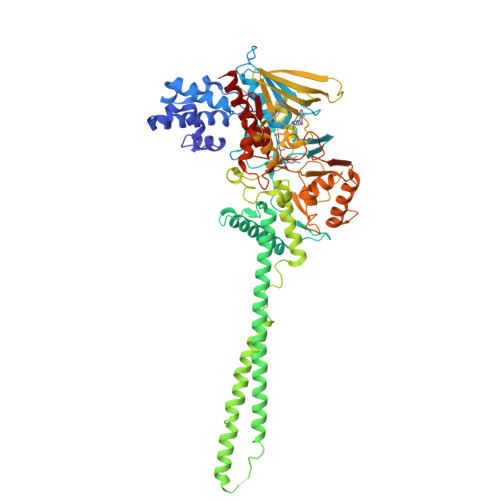
Crystal structure of histone demethylase LSD1 and tranylcypromine at 2.25A
Mimasu, S., Sengoku, T., Fukuzawa, S., Umehara, T., Yokoyama, S.(2008) Biochem Biophys Res Commun 366: 15-22
- PubMed: 18039463
- DOI: https://doi.org/10.1016/j.bbrc.2007.11.066
- Primary Citation of Related Structures:
2DW4, 2EJR, 2Z3Y, 2Z5U - PubMed Abstract:
Transcriptional activity and chromatin structure accessibility are correlated with the methylation of specific histone residues. Lysine-specific demethylase 1 (LSD1) is the first discovered histone demethylase, which demethylates Lys4 or Lys9 of histone H3, using FAD. Among the known monoamine oxidase inhibitors, tranylcypromine (Parnate) showed the most potent inhibitory effect on LSD1. Recently, the crystal structure of LSD1 and tranylcypromine was solved at 2.75 A, revealing a five-membered ring fused to the flavin of LSD1. In this study, we refined the crystal structure of the LSD1-tranylcypromine complex to 2.25 A. The five-membered ring model did not fit completely with the electron density, giving R(work)/R(free) values of 0.226/0.254. On the other hand, the N(5) adduct gave the lowest R(work)/R(free) values of 0.218/0.248, among the tested models. These results imply that the LSD1-tranylcypromine complex is not completely composed of the five-membered adduct, but partially contains an intermediate, such as the N(5) adduct.
Organizational Affiliation:
Department of Biophysics and Biochemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.