Structural Characterization and Epitope Mapping of the Glutamic Acid/Alanine-Rich Protein from Trypanosoma Congolense: Defining Assembly on the Parasite Cell Surface.
Loveless, B.C., Mason, J.W., Sakurai, T., Inoue, N., Razavi, M., Pearson, T.W., Boulanger, M.J.(2011) J Biol Chem 286: 20658
- PubMed: 21471223
- DOI: https://doi.org/10.1074/jbc.M111.218941
- Primary Citation of Related Structures:
2Y44 - PubMed Abstract:
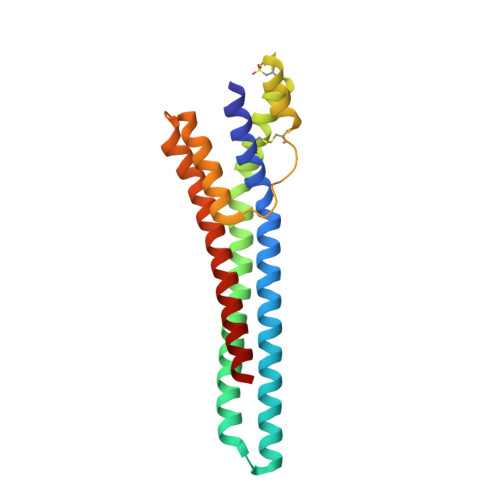
Trypanosoma congolense is an African trypanosome that causes serious disease in cattle in Sub-Saharan Africa. The four major life cycle stages of T. congolense can be grown in vitro, which has led to the identification of several cell-surface molecules expressed on the parasite during its transit through the tsetse vector. One of these, glutamic acid/alanine-rich protein (GARP), is the first expressed on procyclic forms in the tsetse midgut and is of particular interest because it replaces the major surface coat molecule of bloodstream forms, the variant surface glycoprotein (VSG) that protects the parasite membrane, and is involved in antigenic variation. Unlike VSG, however, the function of GARP is not known, which necessarily limits our understanding of parasite survival in the tsetse. Toward establishing the function of GARP, we report its three-dimensional structure solved by iodide phasing to a resolution of 1.65 Å. An extended helical bundle structure displays an unexpected and significant degree of homology to the core structure of VSG, the only other major surface molecule of trypanosomes to be structurally characterized. Immunofluorescence microscopy and immunoaffinity-tandem mass spectrometry were used in conjunction with monoclonal antibodies to map both non-surface-disposed and surface epitopes. Collectively, these studies enabled us to derive a model describing the orientation and assembly of GARP on the surface of trypanosomes. The data presented here suggest the possible structure-function relationships involved in replacement of the bloodstream form VSG by GARP as trypanosomes differentiate in the tsetse vector after a blood meal.
Organizational Affiliation:
Department of Biochemistry and Microbiology, University of Victoria, Victoria, British Columbia V8W 3P6, Canada.