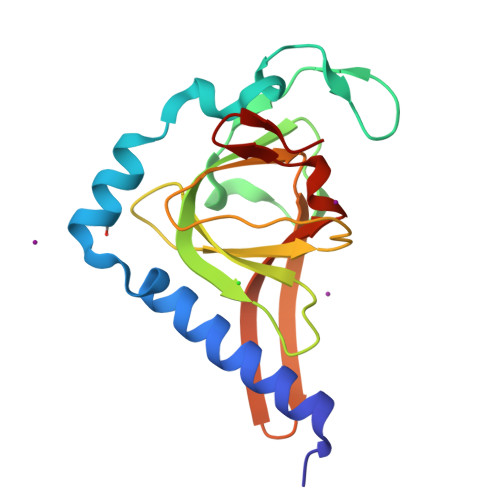
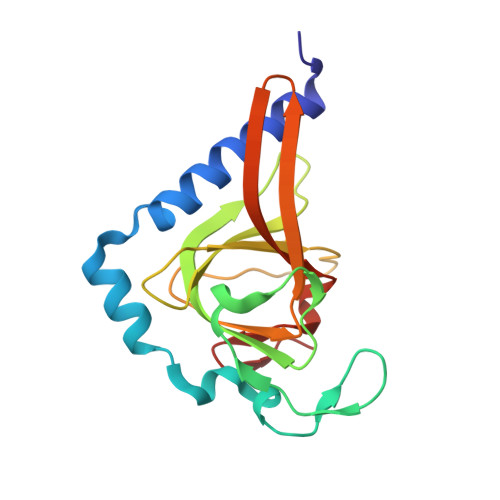
The P53 Cofactor Strap Exhibits an Unexpected Tpr Motif and Oligonucleotide-Binding (Ob)-Fold Structure.
Adams, C.J., Pike, A.C., Maniam, S., Sharpe, T.D., Coutts, A.S., Knapp, S., La Thangue, N.B., Bullock, A.N.(2012) Proc Natl Acad Sci U S A 109: 3778
- PubMed: 22362889
- DOI: https://doi.org/10.1073/pnas.1113731109
- Primary Citation of Related Structures:
2XVS, 4ABN - PubMed Abstract:
Activation of p53 target genes for tumor suppression depends on the stress-specific regulation of transcriptional coactivator complexes. Strap (stress-responsive activator of p300) is activated upon DNA damage by ataxia telangiectasia mutated (ATM) and Chk2 kinases and is a key regulator of the p53 response. In addition to antagonizing Mdm2, Strap facilitates the recruitment of p53 coactivators, including JMY and p300. Strap is a predicted TPR-repeat protein, but shows only limited sequence identity with any protein of known structure. To address this and to elucidate the molecular mechanism of Strap activity we determined the crystal structure of the full-length protein at 2.05 Å resolution. The structure of Strap reveals an atypical six tetratricopeptide repeat (TPR) protein that also contains an unexpected oligonucleotide/oligosaccharide-binding (OB)-fold domain. This previously unseen domain organization provides an extended superhelical scaffold allowing for protein-protein as well as protein-DNA interaction. We show that both of the TPR and OB-fold domains localize to the chromatin of p53 target genes and exhibit intrinsic regulatory activity necessary for the Strap-dependent p53 response.
Organizational Affiliation:
Laboratory of Cancer Biology, Department of Oncology, Medical Sciences Division, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford, OX3 7DQ, United Kingdom.