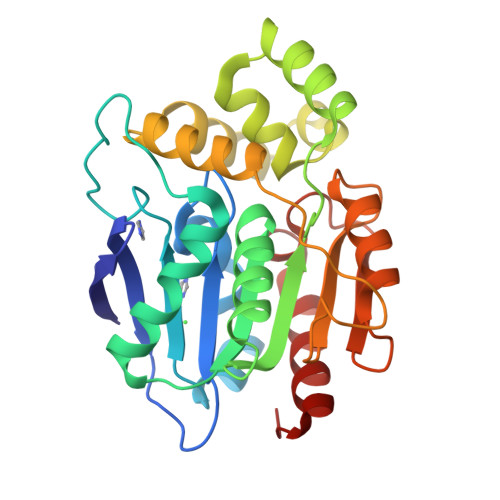
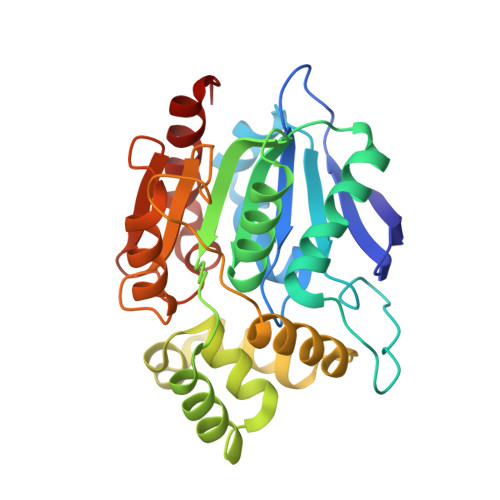
Crystal Structure of Human Ndrg2 Protein Provides Insight Into its Role as a Tumor Suppressor.
Hwang, J., Kim, Y., Kang, H.B., Jaroszewski, L., Deacon, A., Lee, H., Choi, W.C., Kim, K.J., Kim, C.H., Kang, B.S., Lee, J.O., Oh, T.K., Kim, J.W., Wilson, I.A., Kim, M.H.(2011) J Biological Chem 286: 12450
- PubMed: 21247902
- DOI: https://doi.org/10.1074/jbc.M110.170803
- Primary Citation of Related Structures:
2QMQ, 2XMQ, 2XMR, 2XMS - PubMed Abstract:
Considerable attention has recently been paid to the N-Myc downstream-regulated gene (NDRG) family because of its potential as a tumor suppressor in many human cancers. Primary amino acid sequence information suggests that the NDRG family proteins may belong to the α/β-hydrolase (ABH) superfamily; however, their functional role has not yet been determined. Here, we present the crystal structures of the human and mouse NDRG2 proteins determined at 2.0 and 1.7 Å resolution, respectively. Both NDRG2 proteins show remarkable structural similarity to the ABH superfamily, despite limited sequence similarity. Structural analysis suggests that NDRG2 is a nonenzymatic member of the ABH superfamily, because it lacks the catalytic signature residues and has an occluded substrate-binding site. Several conserved structural features suggest NDRG may be involved in molecular interactions. Mutagenesis data based on the structural analysis support a crucial role for helix α6 in the suppression of TCF/β-catenin signaling in the tumorigenesis of human colorectal cancer, via a molecular interaction.
Organizational Affiliation:
Division of Biosystems Research, Korea Research Institute of Bioscience and Biotechnology, Daejeon 305-806, Korea.