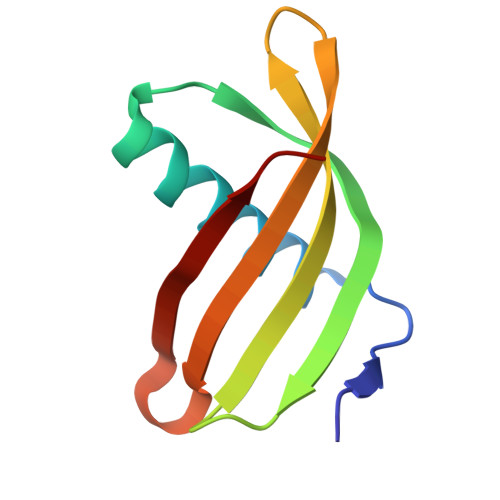
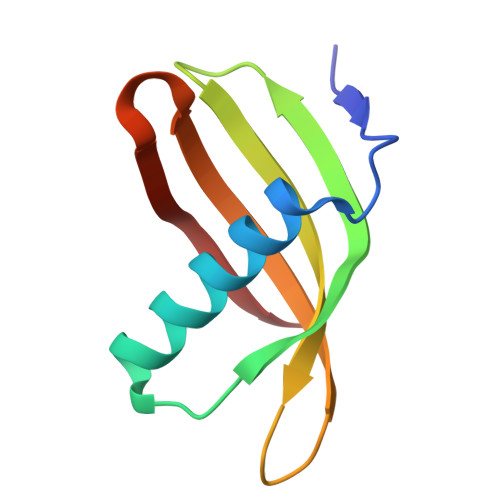
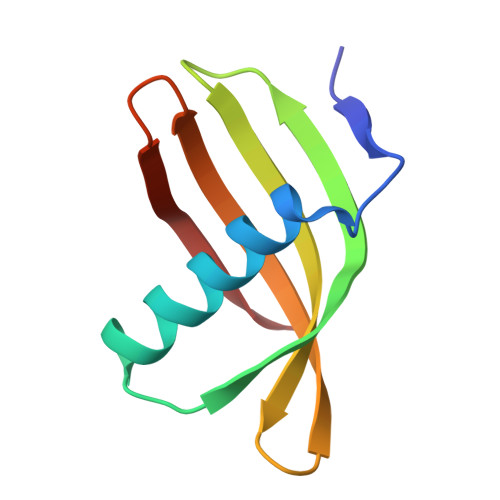
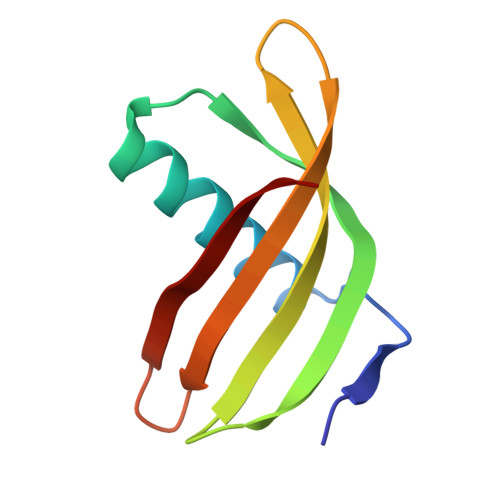
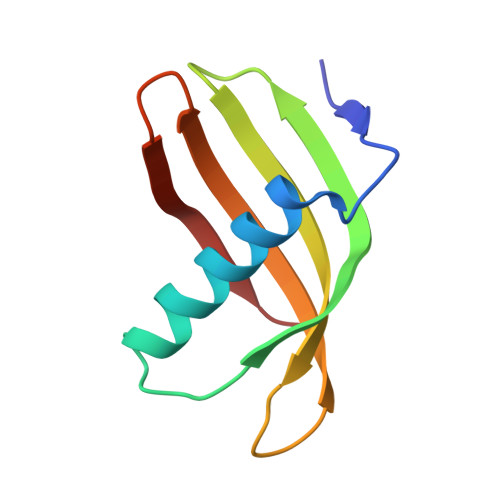
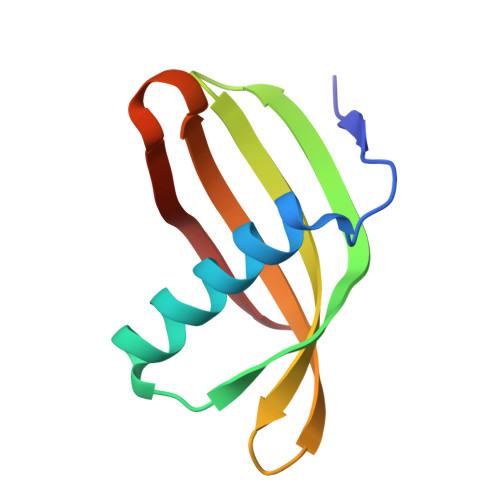
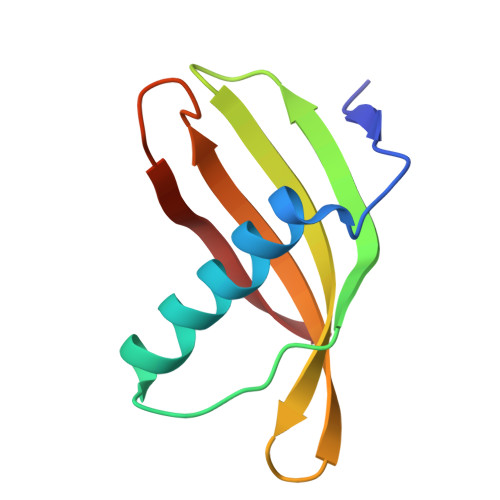
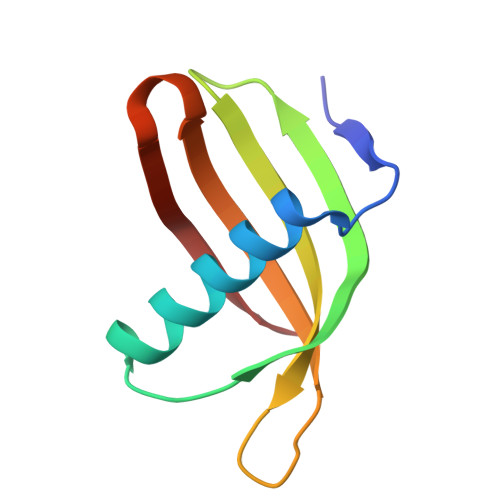
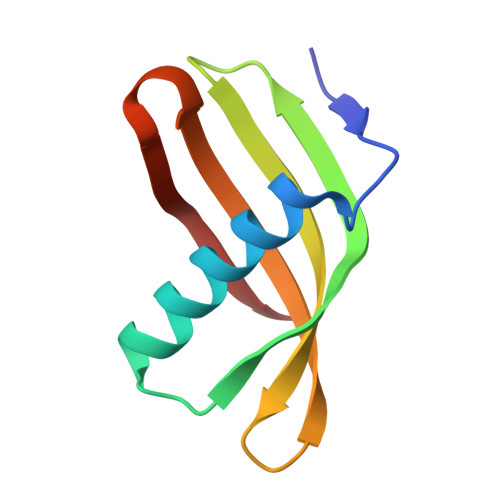
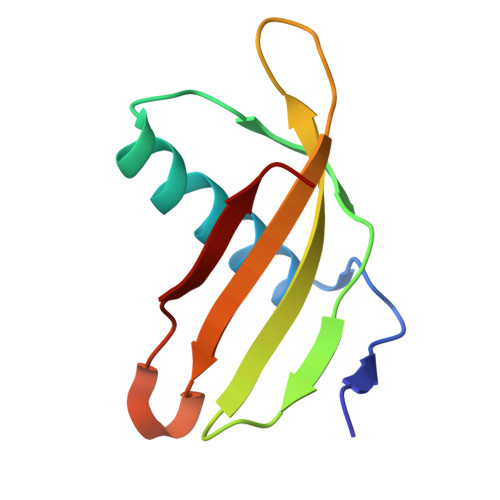
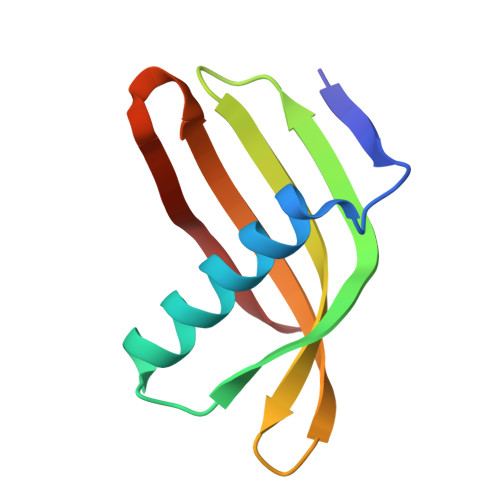
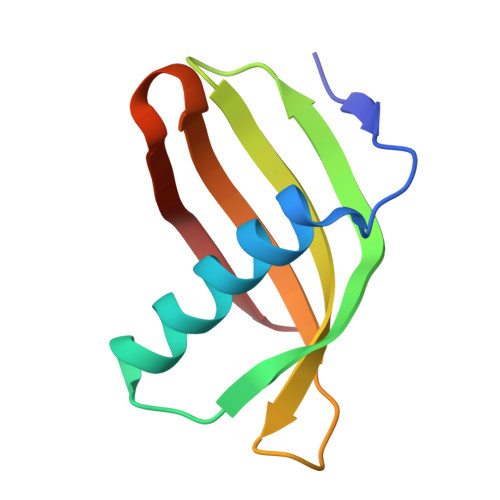
Characterization of Solanum Tuberosum Multicystatin and its Structural Comparison with Other Cystatins.
Nissen, M.S., Kumar, G.N., Youn, B., Knowles, D.B., Lam, K.S., Ballinger, W.J., Knowles, N.R., Kang, C.(2009) Plant Cell 21: 861
- PubMed: 19304935
- DOI: https://doi.org/10.1105/tpc.108.064717
- Primary Citation of Related Structures:
2W9P, 2W9Q - PubMed Abstract:
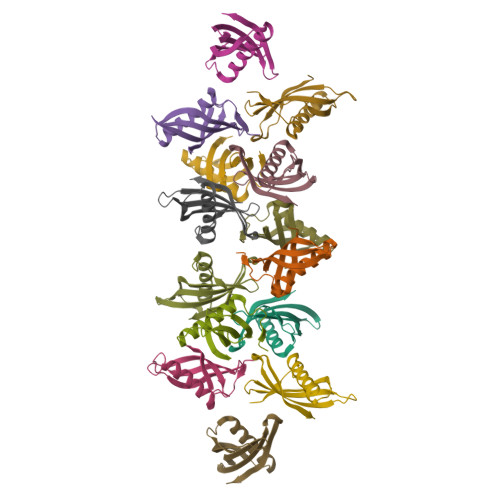
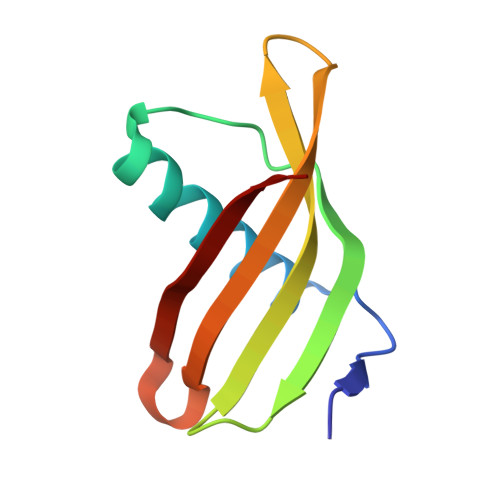
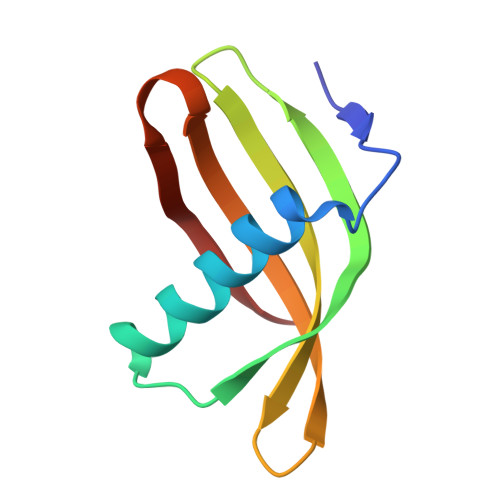
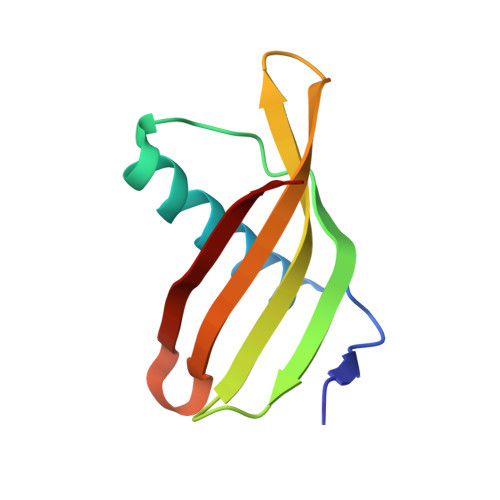
Potato (Solanum tuberosum) multicystatin (PMC) is a crystalline Cys protease inhibitor present in the subphellogen layer of potato tubers. It consists of eight tandem domains of similar size and sequence. Our in vitro results showed that the pH/PO(4)(-)-dependent oligomeric behavior of PMC was due to its multidomain nature and was not a characteristic of the individual domains. Using a single domain of PMC, which still maintains inhibitor activity, we identified a target protein of PMC, a putative Cys protease. In addition, our crystal structure of a representative repeating unit of PMC, PMC-2, showed structural similarity to both type I and type II cystatins. The N-terminal trunk, alpha-helix, and L2 region of PMC-2 were most similar to those of type I cystatins, while the conformation of L1 more closely resembled that of type II cystatins. The structure of PMC-2 was most similar to the intensely sweet protein monellin from Dioscorephyllum cumminisii (serendipity berry), despite a low level of sequence similarity. We present a model for the possible molecular organization of the eight inhibitory domains in crystalline PMC. The unique molecular properties of the oligomeric PMC crystal are discussed in relation to its potential function in regulating the activity of proteases in potato tubers.
Organizational Affiliation:
School of Molecular Biosciences, Washington State University, Pullman, Washington 99164-4660, USA.