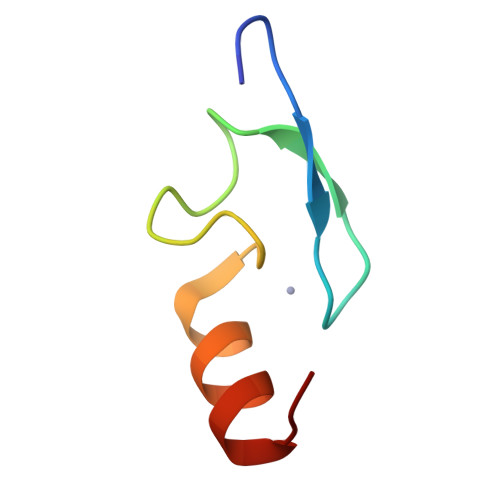
Solution Structure of the Fcs Zinc Finger Domain of the Human Polycomb Group Protein L(3)Mbt-Like 2.
Lechtenberg, B.C., Allen, M.D., Rutherford, T.J., Freund, S.M., Bycroft, M.(2009) Protein Sci 18: 657
- PubMed: 19241375
- DOI: https://doi.org/10.1002/pro.51
- Primary Citation of Related Structures:
2W0T - PubMed Abstract:
Polycomb group proteins are epigenetic regulators that maintain patterns of gene expression over multiple rounds of cell division. Many of these proteins, including polyhomeotic and the MBT repeat containing proteins SCM and dSfmbt, contain an atypical C2C2 zinc finger with a characteristic phenylalanine-cysteine-serine sequence motif. The reoccurrence of this so-called FCS zinc finger in a variety of polycomb group proteins suggests that it has an important regulatory function. We have determined the solution structure of the FCS zinc finger of the human dSfmbt homologue L(3)mbt-like 2 (L3MBTL2). The structure consists of a beta-hairpin followed by an alpha-helix. The zinc ligands are situated in the beta-hairpin and at the N-terminus of the alpha-helix an arrangement typical of the treble clef class of zinc fingers. The structure is consistent with the proposal that FCS zinc fingers bind to regulatory RNAs.
Organizational Affiliation:
MRC Centre for Protein Engineering, Cambridge, United Kingdom.