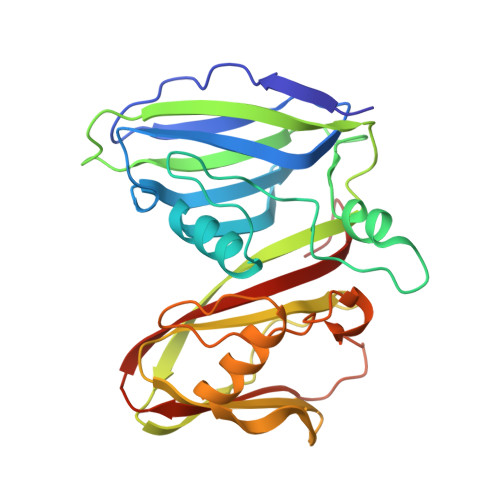
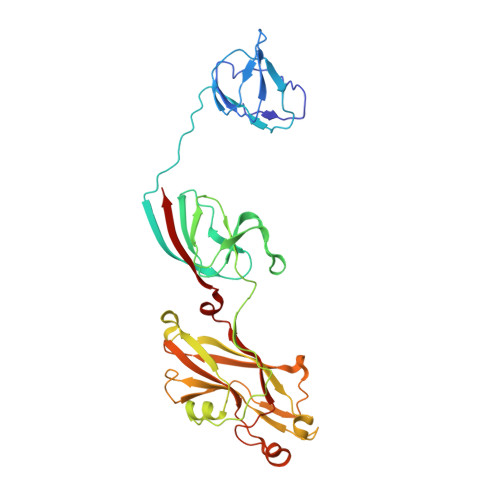
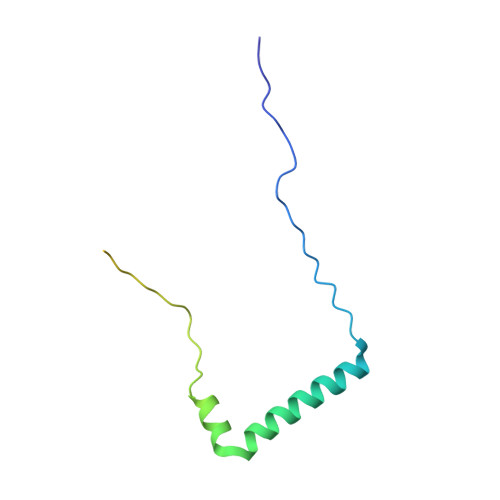
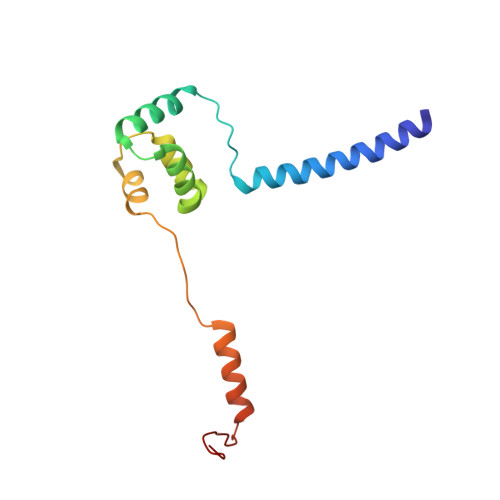
Insights Into Virus Evolution and Membrane Biogenesis from the Structure of the Marine Lipid-Containing Bacteriophage Pm2
Abrescia, N.G.A., Grimes, J.M., Kivela, H.M., Assenberg, R., Sutton, G.C., Butcher, S.J., Bamford, J.K.H., Bamford, D.H., Stuart, D.I.(2008) Mol Cell 31: 749
- PubMed: 18775333
- DOI: https://doi.org/10.1016/j.molcel.2008.06.026
- Primary Citation of Related Structures:
2VVD, 2VVE, 2VVF, 2W0C - PubMed Abstract:
Recent, primarily structural observations indicate that related viruses, harboring no sequence similarity, infect hosts of different domains of life. One such clade of viruses, defined by common capsid architecture and coat protein fold, is the so-called PRD1-adenovirus lineage. Here we report the structure of the marine lipid-containing bacteriophage PM2 determined by crystallographic analyses of the entire approximately 45 MDa virion and of the outer coat proteins P1 and P2, revealing PM2 to be a primeval member of the PRD1-adenovirus lineage with an icosahedral shell and canonical double beta barrel major coat protein. The view of the lipid bilayer, richly decorated with membrane proteins, constitutes a rare visualization of an in vivo membrane. The viral membrane proteins P3 and P6 are organized into a lattice, suggesting a possible assembly pathway to produce the mature virus.
- Division of Structural Biology and the Oxford Protein Production Facility, The Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Headington, Oxford OX3 7BN, UK.
Organizational Affiliation: