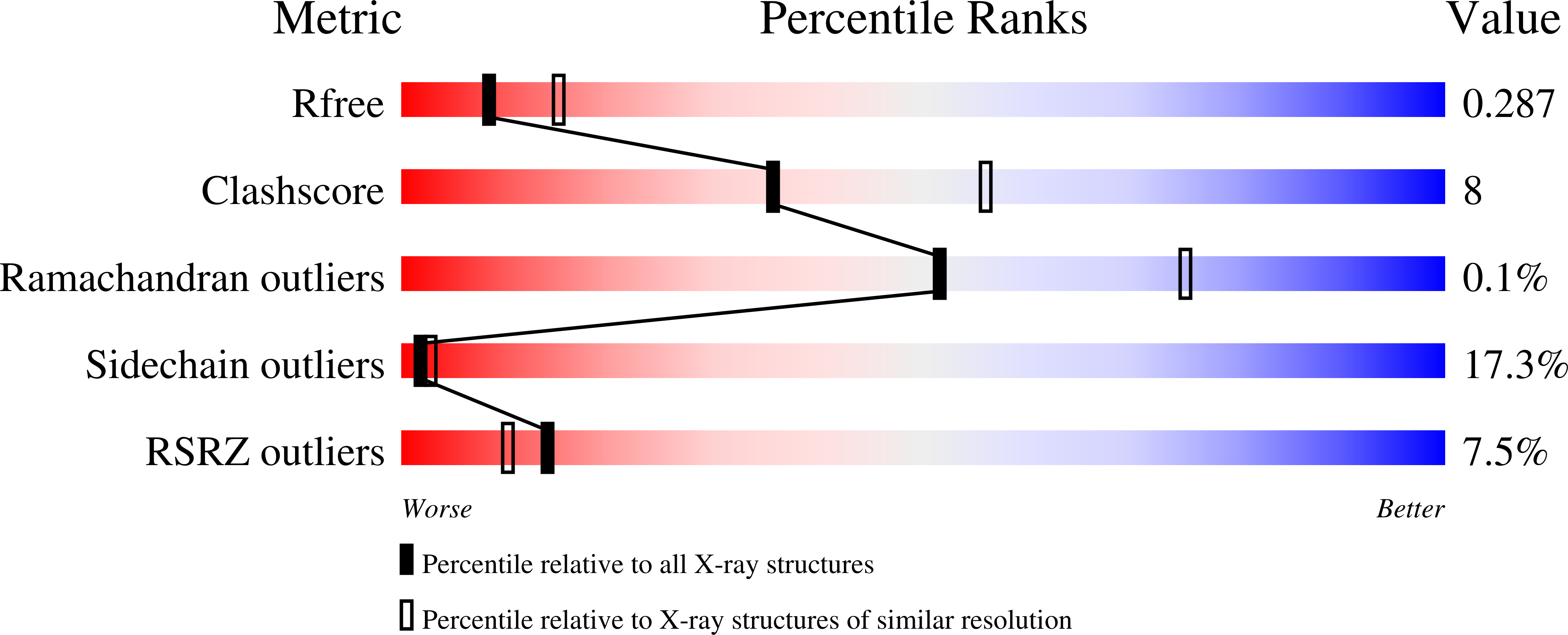
Dodecin is the Key Player in Flavin Homeostasis of Archaea.
Grininger, M., Staudtb, H., Johansson, P., Wachtveitl, J., Oesterhelt, D.(2009) J Biol Chem 284: 13068
- PubMed: 19224924
- DOI: https://doi.org/10.1074/jbc.M808063200
- Primary Citation of Related Structures:
2VX9, 2VXA - PubMed Abstract:
Flavins are employed to transform physical input into biological output signals. In this function, flavins catalyze a variety of light-induced reactions and redox processes. However, nature also provides flavoproteins with the ability to uncouple the mediation of signals. Such proteins are the riboflavin-binding proteins (RfBPs) with their function to store riboflavin for fast delivery of FMN and FAD. Here we present in vitro and in vivo data showing that the recently discovered archaeal dodecin is an RfBP, and we reveal that riboflavin storage is not restricted to eukaryotes. However, the function of the prokaryotic RfBP dodecin seems to be adapted to the requirement of a monocellular organism. While in eukaryotes RfBPs are involved in trafficking riboflavin, and dodecin is responsible for the flavin homeostasis of the cell. Although only 68 amino acids in length, dodecin is of high functional versatility in neutralizing riboflavin to protect the cellular environment from uncontrolled flavin reactivity. Besides the predominant ultrafast quenching of excited states, dodecin prevents light-induced riboflavin reactivity by the selective degradation of riboflavin to lumichrome. Coordinated with the high affinity for lumichrome, the directed degradation reaction is neutral to the cellular environment and provides an alternative pathway for suppressing uncontrolled riboflavin reactivity. Intriguingly, the different structural and functional properties of a homologous bacterial dodecin suggest that dodecin has different roles in different kingdoms of life.
Organizational Affiliation:
Department of Membrane Biochemistry, Max Planck Institute of Biochemistry, Martinsried, Germany. grininge@biochem.mpg.de