Structural and Functional Dissection of Mif2P, a Conserved DNA-Binding Kinetochore Protein.
Cohen, R.L., Espelin, C.W., De Wulf, P., Sorger, P.K., Harrison, S.C., Simons, K.T.(2008) Mol Biol Cell 19: 4480
- PubMed: 18701705
- DOI: https://doi.org/10.1091/mbc.e08-03-0297
- Primary Citation of Related Structures:
2VPV - PubMed Abstract:
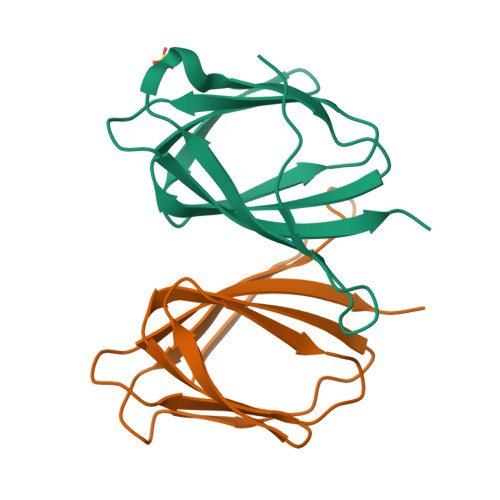
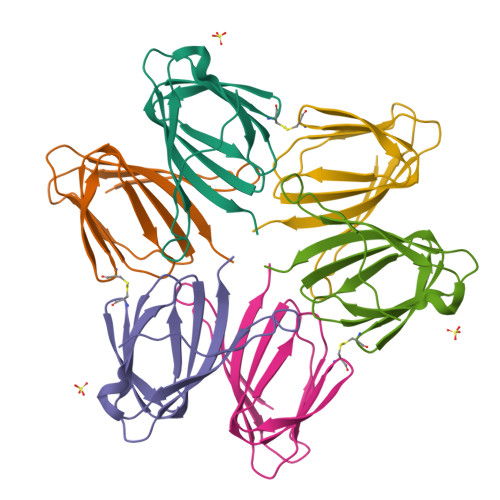
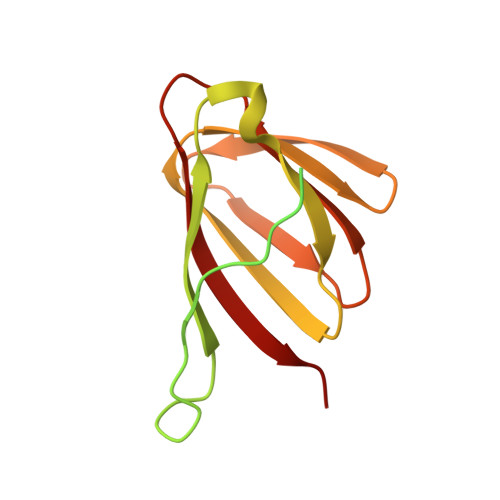
Mif2p is the budding-yeast orthologue of the mammalian centromere-binding protein CENP-C. We have mapped domains of Saccharomyces cerevisiae Mif2p and studied the phenotyptic consequences of their deletion. Using chromatin immunoprecipitation (ChIP) and electrophoretic mobility shift assays, we have further shown that Mif2p binds in the CDEIII region of the budding-yeast centromere, probably in close spatial association with Ndc10p. Moreover, ChIP experiments show that Mif2p recruits to yeast kinetochores a substantial subset of inner and outer kinetochore proteins, but not the Ndc80 or Spc105 complexes. We have determined the crystal structure of the C-terminal, dimerization domain of Mif2p. It has a "cupin" fold, extremely similar both in polypeptide chain conformation and in dimer geometry to the dimerization domain of a bacterial transcription factor. The Mif2p dimer seems to be part of an enhanceosome-like structure that nucleates kinetochore assembly in budding yeast.
Organizational Affiliation:
Jack and Eileen Connors Structural Biology Laboratory, Harvard Medical School, Boston, MA 02115, USA.