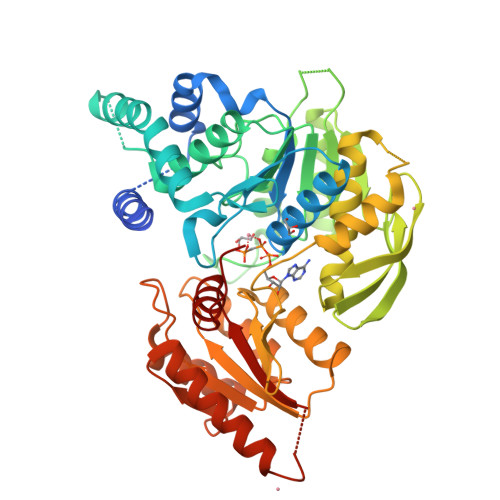
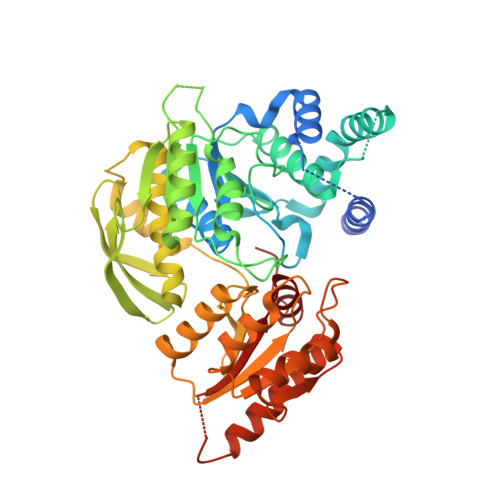
Structures of Mycobacterium Tuberculosisfolylpolyglutamate Synthase Complexed with Adp and Amppcp.
Young, P.G., Smith, C.A., Metcalf, P., Baker, E.N.(2008) Acta Crystallogr D Biol Crystallogr 64: 745
- PubMed: 18566510
- DOI: https://doi.org/10.1107/S0907444908012262
- Primary Citation of Related Structures:
2VOR, 2VOS - PubMed Abstract:
Folate derivatives are essential vitamins for cell growth and replication, primarily because of their central role in reactions of one-carbon metabolism. Folates require polyglutamation to be efficiently retained within the cell and folate-dependent enzymes have a higher affinity for the polyglutamylated forms of this cofactor. Polyglutamylation is dependent on the enzyme folylpolyglutamate synthetase (FPGS), which catalyzes the sequential addition of several glutamates to folate. FPGS is essential for the growth and survival of important bacterial species, including Mycobacterium tuberculosis, and is a potential drug target. Here, the crystal structures of M. tuberculosis FPGS in complex with ADP and AMPPCP are reported at 2.0 and 2.3 angstroms resolution, respectively. The structures reveal a deeply buried nucleotide-binding site, as in the Escherichia coli and Lactobacillus casei FPGS structures, and a long extended groove for the binding of folate substrates. Differences from the E. coli and L. casei FPGS structures are seen in the binding of a key divalent cation, the carbamylation state of an essential lysine side chain and the adoption of an 'open' position by the active-site beta5-alpha6 loop. These changes point to coordinated events that are associated with dihydropteroate/folate binding and the catalysis of the new amide bond with an incoming glutamate residue.
Organizational Affiliation:
School of Biological Sciences, University of Auckland, Auckland, New Zealand. p.young@auckland.ac.nz