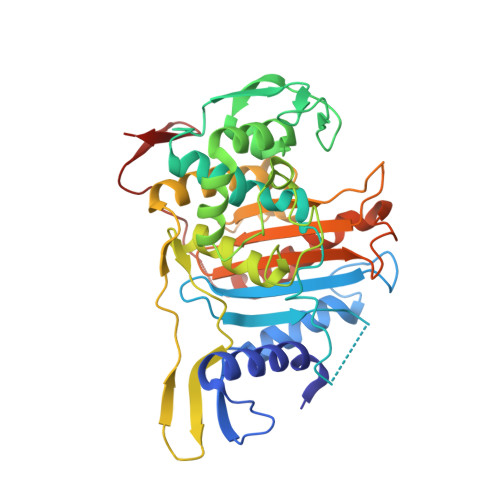
Common Alterations in Pbp1A from Resistant Streptococcus Pneumoniae Decrease its Reactivity Toward {Beta}-Lactams: Structural Insights.
Job, V., Carapito, R., Vernet, T., Dessen, A., Zapun, A.(2008) J Biol Chem 283: 4886
- PubMed: 18055459
- DOI: https://doi.org/10.1074/jbc.M706181200
- Primary Citation of Related Structures:
2V2F - PubMed Abstract:
The development of high level beta-lactam resistance in the pneumococcus requires the expression of an altered form of PBP1a, in addition to modified forms of PBP2b and PBP2x, which are necessary for the appearance of low levels of resistance. Here, we present the crystal structure of a soluble form of PBP1a from the highly resistant Streptococcus pneumoniae strain 5204 (minimal inhibitory concentration of cefotaxime is 12 mg.liter(-1)). Mutations T371A, which is adjacent to the catalytic nucleophile Ser370, and TSQF(574-577)NTGY, which lie in a loop bordering the active site cleft, were investigated by site-directed mutagenesis. The consequences of these substitutions on reaction kinetics with beta-lactams were probed in vitro, and their effect on resistance was measured in vivo. The results are interpreted in the framework of the crystal structure, which displays a narrower, discontinuous active site cavity, compared with that of PBP1a from the beta-lactam susceptible strain R6, as well as a reorientation of the catalytic Ser370.
Organizational Affiliation:
Laboratoire des Protéines Membranaires, Institut de Biologie Structurale Jean-Pierre Ebel, Université Joseph Fourier, UMR 5075-CNRS, CEA Grenoble, France.