The Dodecin from Thermus Thermophilus, a Bifunctional Cofactor Storage Protein.
Meissner, B., Schleicher, E., Weber, S., Essen, L.-O.(2007) J Biological Chem 282: 33142
- PubMed: 17855371
- DOI: https://doi.org/10.1074/jbc.M704951200
- Primary Citation of Related Structures:
2UX9, 2V18, 2V19, 2V21 - PubMed Abstract:
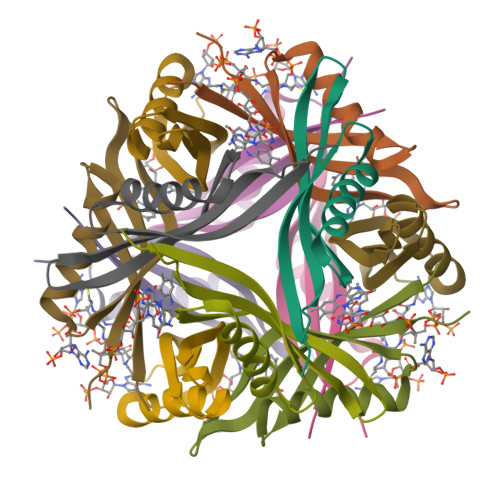
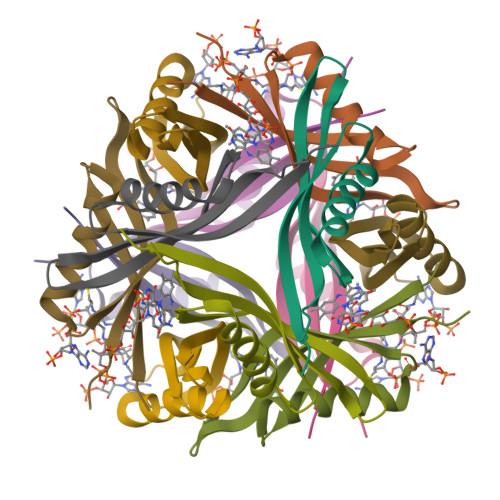
Dodecins are so far the smallest known flavoproteins (68-71 amino acids) and are most likely involved in prokaryotic flavin storage. The dodecin monomers adopt a simple betaalphabetabeta-fold and assemble to hollow sphere-like dodecameric complexes. Flavin binding by the dodecin from Thermus thermophilus showed a 1:1 stoichiometry and apparent dissociation constants in the submicromolar to nanomolar range as characterized by isothermal titration calorimetry and fluorescence titrations. The x-ray structures of the flavin-prebound and FMN-reconstituted state of the T. thermophilus dodecin revealed binding of FMN dimers in a novel si-si- rather than the re-re- orientation of their isoalloxazine moieties as found before in an archaeal dodecin. Electron paramagnetic resonance studies demonstrated that upon reduction the excess electron is localized only on one flavin, thus making dodecin-bound flavins highly refractory to redox chemistry. Besides FMN dimers, trimers of coenzyme A are additionally bound to this eubacterial dodecin along the 3-fold symmetry face II of the dodecin complex. Therefore, dodecins can act as bifunctional cofactor storage proteins that sequester catalytic cofactors in prokaryotes very efficiently in an aggregated and unreactive state.
Organizational Affiliation:
Department of Chemistry, Philipps-Universität Marburg, Hans-Meerwein-Strasse, Marburg, Germany.