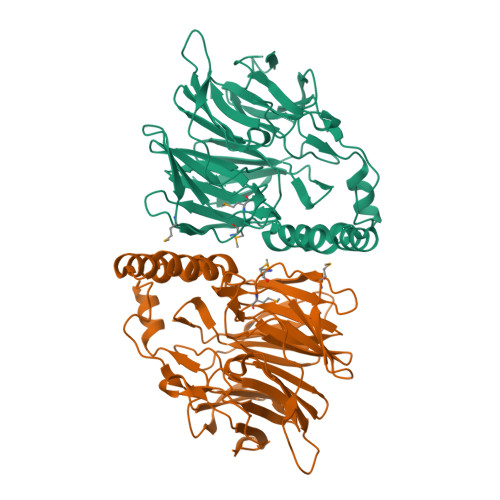
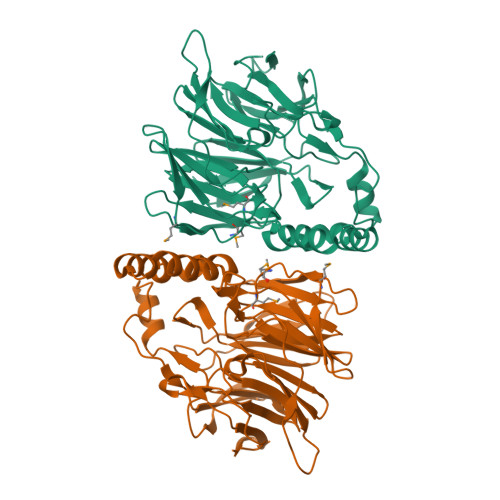
Sialic Acid Mutarotation is Catalyzed by the Escherichia Coli Beta-Propeller Protein Yjht.
Severi, E., Muller, A., Potts, J.R., Leech, A., Williamson, D., Wilson, K.S., Thomas, G.H.(2008) J Biological Chem 283: 4841
- PubMed: 18063573
- DOI: https://doi.org/10.1074/jbc.M707822200
- Primary Citation of Related Structures:
2UVK - PubMed Abstract:
The acquisition of host-derived sialic acid is an important virulence factor for some bacterial pathogens, but in vivo this sugar acid is sequestered in sialoconjugates as the alpha-anomer. In solution, however, sialic acid is present mainly as the beta-anomer, formed by a slow spontaneous mutarotation. We studied the Escherichia coli protein YjhT as a member of a family of uncharacterized proteins present in many sialic acid-utilizing pathogens. This protein is able to accelerate the equilibration of the alpha- and beta-anomers of the sialic acid N-acetylneuraminic acid, thus describing a novel sialic acid mutarotase activity. The structure of this periplasmic protein, solved to 1.5A resolution, reveals a dimeric 6-bladed unclosed beta-propeller, the first of a bacterial Kelch domain protein. Mutagenesis of conserved residues in YjhT demonstrated an important role for Glu-209 and Arg-215 in mutarotase activity. We also present data suggesting that the ability to utilize alpha-N-acetylneuraminic acid released from complex sialoconjugates in vivo provides a physiological advantage to bacteria containing YjhT.
Organizational Affiliation:
Department of Biology (Area 10), York Structural Biology Laboratory, University of York, York YO10 5YW, United Kingdom.