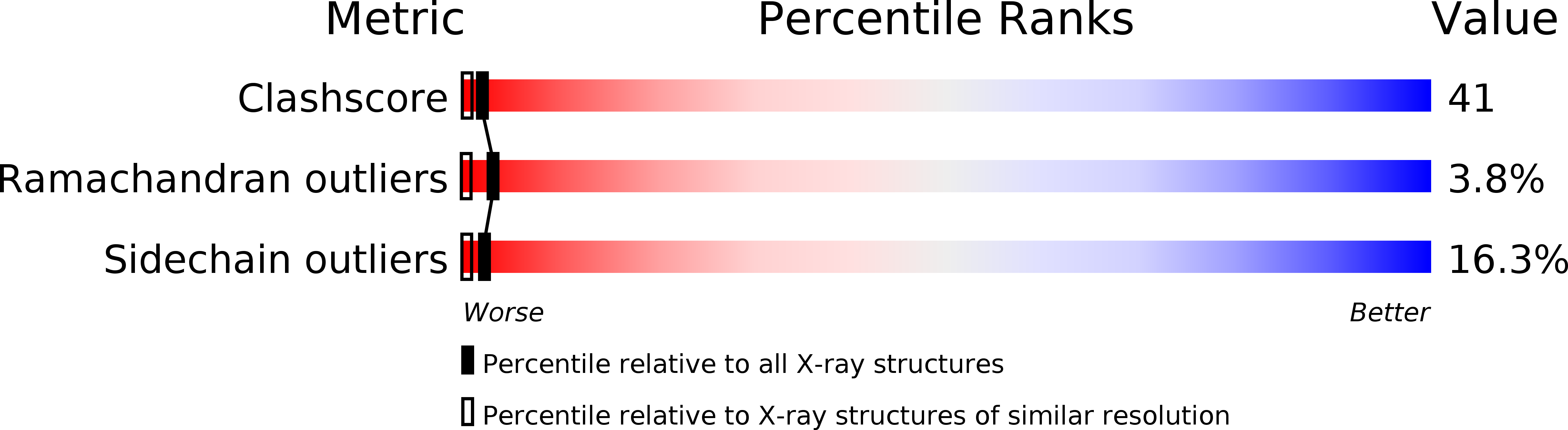Crystal structure of the repetitive segments of spectrin.
Yan, Y., Winograd, E., Viel, A., Cronin, T., Harrison, S.C., Branton, D.(1993) Science 262: 2027-2030
- PubMed: 8266097
- DOI: https://doi.org/10.1126/science.8266097
- Primary Citation of Related Structures:
2SPC - PubMed Abstract:
The elongated proteins of the spectrin family (dystrophin, alpha-actinin, and spectrin) contain tandemly repeated segments and form resilient cellular meshworks by cross-linking actin filaments. The structure of one of the repetitive segments of alpha-spectrin was determined at a 1.8 angstrom resolution. A segment consists of a three-helix bundle. A model of the interface between two tandem segments suggests that hydrophobic interactions between segments may constrain intersegment flexibility. The helix side chain interactions explain how mutations that are known to produce hemolytic anemias disrupt spectrin associations that sustain the integrity of the erythrocyte membrane.
Organizational Affiliation:
Department of Biochemistry, Harvard University, Cambridge, MA 02138.