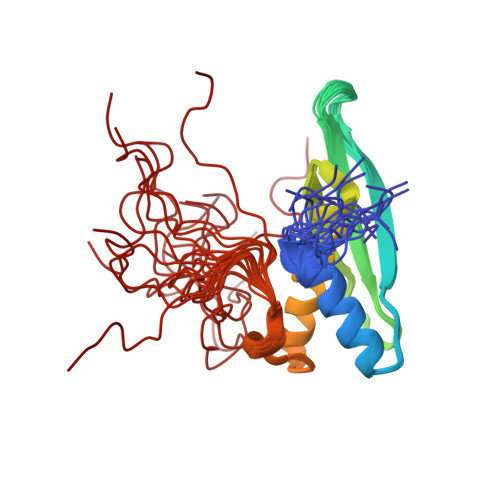
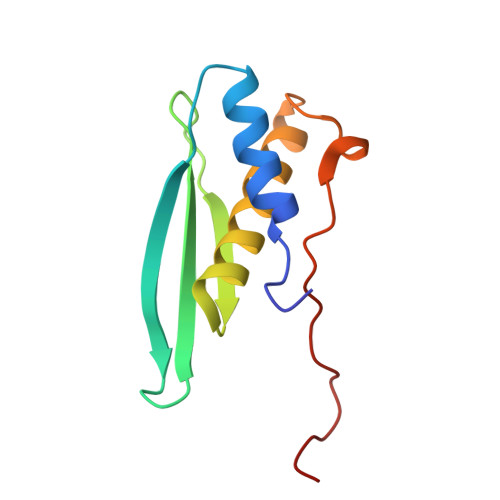
Solution structures of the double-stranded RNA-binding domains from RNA helicase A
Nagata, T., Tsuda, K., Kobayashi, N., Shirouzu, M., Kigawa, T., Guntert, P., Yokoyama, S., Muto, Y.(2012) Proteins 80: 1699-1706
- PubMed: 22454253
- DOI: https://doi.org/10.1002/prot.24059
- Primary Citation of Related Structures:
2RS6, 2RS7 - PubMed Abstract:
RNA helicase A (RHA) is a highly conserved protein with multifaceted functions in the gene expression of cellular and viral mRNAs. RHA recognizes highly structured nucleotides and catalytically rearranges the various interactions between RNA, DNA, and protein molecules to provide a platform for the ribonucleoprotein complex. We present the first solution structures of the double-stranded RNA-binding domains (dsRBDs), dsRBD1 and dsRBD2, from mouse RHA. We discuss the binding mode of the dsRBDs of RHA, in comparison with the known dsRBD structures in their complexes. Our structural data provide important information for the elucidation of the molecular reassembly mediated by RHA.
Organizational Affiliation:
Institute of Advanced Energy, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan.