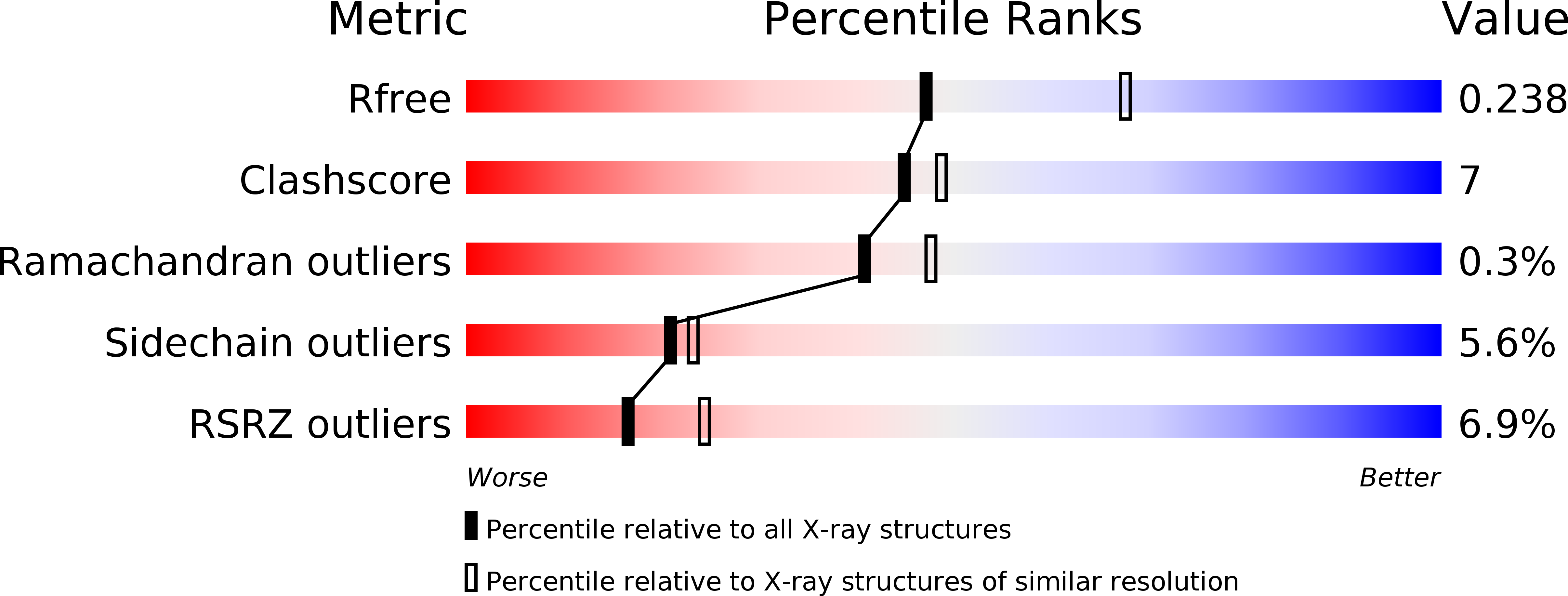
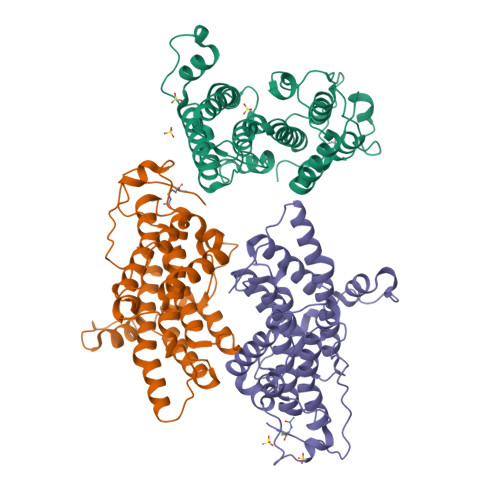
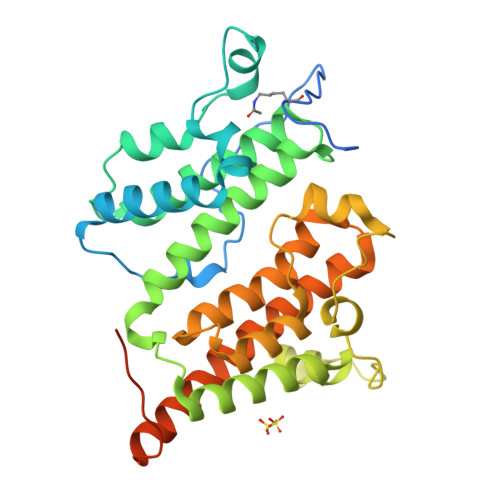
Autoregulation of the rsc4 tandem bromodomain by gcn5 acetylation.
VanDemark, A.P., Kasten, M.M., Ferris, E., Heroux, A., Hill, C.P., Cairns, B.R.(2007) Mol Cell 27: 817-828
- PubMed: 17803945
- DOI: https://doi.org/10.1016/j.molcel.2007.08.018
- Primary Citation of Related Structures:
2R0S, 2R0V, 2R0Y, 2R10 - PubMed Abstract:
An important issue for chromatin remodeling complexes is how their bromodomains recognize particular acetylated lysine residues in histones. The Rsc4 subunit of the yeast remodeler RSC contains an essential tandem bromodomain (TBD) that binds acetylated K14 of histone H3 (H3K14ac). We report a series of crystal structures that reveal a compact TBD that binds H3K14ac in the second bromodomain and, remarkably, binds acetylated K25 of Rsc4 itself in the first bromodomain. Endogenous Rsc4 is acetylated only at K25, and Gcn5 is identified as necessary and sufficient for Rsc4 K25 acetylation in vivo and in vitro. Rsc4 K25 acetylation inhibits binding to H3K14ac, and mutation of Rsc4 K25 results in altered growth rates. These data suggest an autoregulatory mechanism in which Gcn5 performs both the activating (H3K14ac) and inhibitory (Rsc4 K25ac) modifications, perhaps to provide temporal regulation. Additional regulatory mechanisms are indicated as H3S10 phosphorylation inhibits Rsc4 binding to H3K14ac peptides.
Organizational Affiliation:
Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT 84112, USA.