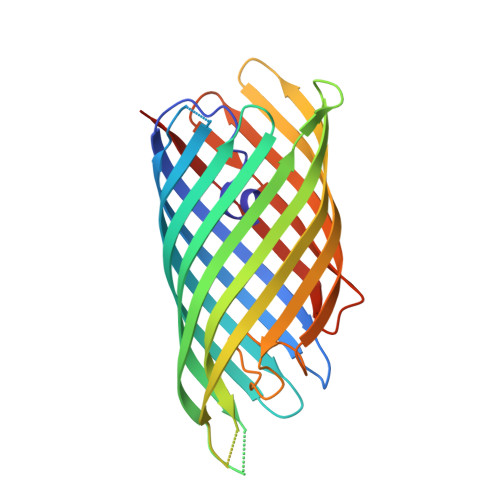
Autotransporter structure reveals intra-barrel cleavage followed by conformational changes.
Barnard, T.J., Dautin, N., Lukacik, P., Bernstein, H.D., Buchanan, S.K.(2007) Nat Struct Mol Biol 14: 1214-1220
- PubMed: 17994105
- DOI: https://doi.org/10.1038/nsmb1322
- Primary Citation of Related Structures:
2QOM - PubMed Abstract:
Autotransporters are virulence factors produced by Gram-negative bacteria. They consist of two domains, an N-terminal 'passenger' domain and a C-terminal beta-domain. beta-domains form beta-barrel structures in the outer membrane while passenger domains are translocated into the extracellular space. In some autotransporters, the two domains are separated by proteolytic cleavage. Using X-ray crystallography, we solved the 2.7-A structure of the post-cleavage state of the beta-domain of EspP, an autotransporter produced by Escherichia coli strain O157:H7. The structure consists of a 12-stranded beta-barrel with the passenger domain-beta-domain cleavage junction located inside the barrel pore, approximately midway between the extracellular and periplasmic surfaces of the outer membrane. The structure reveals an unprecedented intra-barrel cleavage mechanism and suggests that two conformational changes occur in the beta-domain after cleavage, one conferring increased stability on the beta-domain and another restricting access to the barrel pore.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, US National Institutes of Health, Bethesda, Maryland 20892, USA.
Organizational Affiliation: