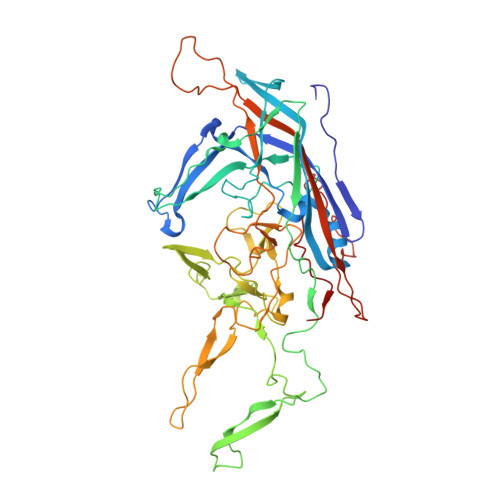
Structure of adeno-associated virus serotype 8, a gene therapy vector.
Nam, H.J., Lane, M.D., Padron, E., Gurda, B., McKenna, R., Kohlbrenner, E., Aslanidi, G., Byrne, B., Muzyczka, N., Zolotukhin, S., Agbandje-McKenna, M.(2007) J Virol 81: 12260-12271
- PubMed: 17728238
- DOI: https://doi.org/10.1128/JVI.01304-07
- Primary Citation of Related Structures:
2QA0 - PubMed Abstract:
Adeno-associated viruses (AAVs) are being developed as gene therapy vectors, and their efficacy could be improved by a detailed understanding of their viral capsid structures. AAV serotype 8 (AAV8) shows a significantly greater liver transduction efficiency than those of other serotypes, which has resulted in efforts to develop this virus as a gene therapy vector for hemophilia A and familial hypercholesterolemia. Pseudotyping studies show that the differential tissue tropism and transduction efficiencies exhibited by the AAVs result from differences in their capsid viral protein (VP) amino acids. Towards identifying the structural features underpinning these disparities, we report the crystal structure of the AAV8 viral capsid determined to 2.6-A resolution. The overall topology of its common overlapping VP is similar to that previously reported for the crystal structures of AAV2 and AAV4, with an eight-stranded beta-barrel and long loops between the beta-strands. The most significant structural differences between AAV8 and AAV2 (the best-characterized serotype) are located on the capsid surface at protrusions surrounding the two-, three-, and fivefold axes at residues reported to control transduction efficiency and antibody recognition for AAV2. In addition, a comparison of the AAV8 and AAV2 capsid surface amino acids showed a reduced distribution of basic charge for AAV8 at the mapped AAV2 heparin sulfate receptor binding region, consistent with an observed non-heparin-binding phenotype for AAV8. Thus, this AAV8 structure provides an additional platform for mutagenesis efforts to characterize AAV capsid regions responsible for differential cellular tropism, transduction, and antigenicity for these promising gene therapy vectors.
- Department of Biochemistry and Molecular Biology, Center for Structural Biology, McKnight Brain Institute, University of Florida, Gainesville, FL 32610, USA.
Organizational Affiliation: