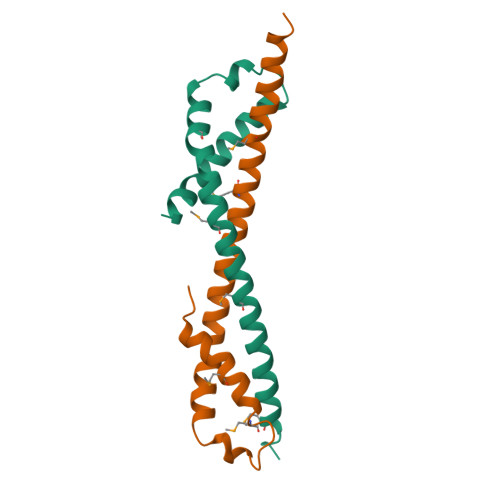
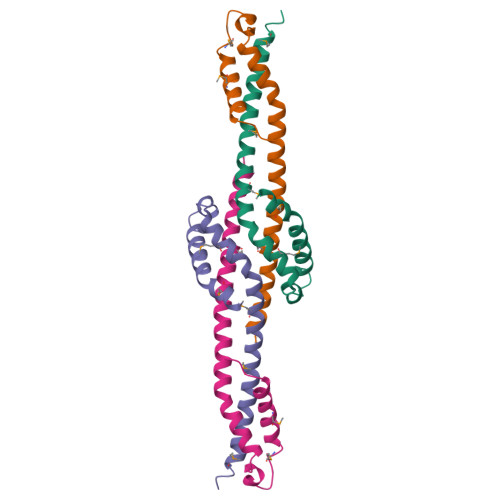
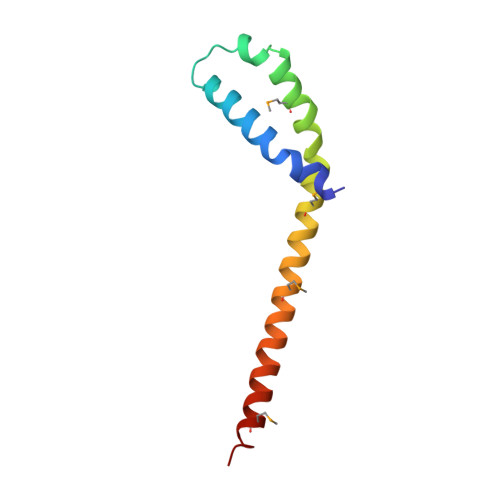
The Vps27/Hse1 Complex Is a GAT Domain-Based Scaffold for Ubiquitin-Dependent Sorting.
Prag, G., Watson, H., Kim, Y.C., Beach, B.M., Ghirlando, R., Hummer, G., Bonifacino, J.S., Hurley, J.H.(2007) Dev Cell 12: 973-986
- PubMed: 17543868
- DOI: https://doi.org/10.1016/j.devcel.2007.04.013
- Primary Citation of Related Structures:
2PJW - PubMed Abstract:
The yeast Vps27/Hse1 complex and the homologous mammalian Hrs/STAM complex deliver ubiquitinated transmembrane proteins to the ESCRT endosomal-sorting pathway. The Vps27/Hse1 complex directly binds to ubiquitinated transmembrane proteins and recruits both ubiquitin ligases and deubiquitinating enzymes. We have solved the crystal structure of the core responsible for the assembly of the Vps27/Hse1 complex at 3.0 A resolution. The structure consists of two intertwined GAT domains, each consisting of two helices from one subunit and one from the other. The two GAT domains are connected by an antiparallel coiled coil, forming a 90 A-long barbell-like structure. This structure places the domains of Vps27 and Hse1 that recruit ubiquitinated cargo and deubiquitinating enzymes close to each other. Coarse-grained Monte Carlo simulations of the Vps27/Hse1 complex on a membrane show how the complex binds cooperatively to lipids and ubiquitinated membrane proteins and acts as a scaffold for ubiquitination reactions.
Organizational Affiliation:
Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, MD 20892, USA.