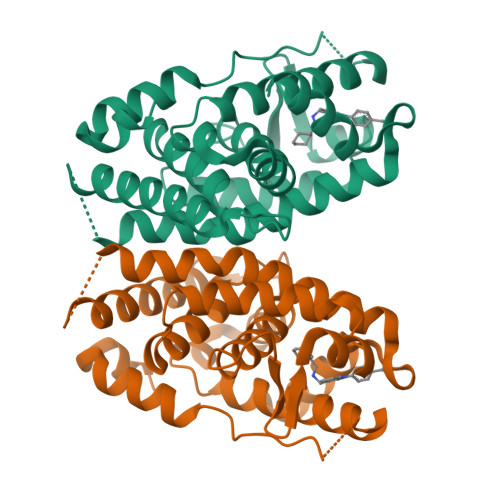
Crystal structure of human estrogen-related receptor alpha in complex with a synthetic inverse agonist reveals its novel molecular mechanism.
Kallen, J., Lattmann, R., Beerli, R., Blechschmidt, A., Blommers, M.J., Geiser, M., Ottl, J., Schlaeppi, J.M., Strauss, A., Fournier, B.(2007) J Biological Chem 282: 23231-23239
- PubMed: 17556356
- DOI: https://doi.org/10.1074/jbc.M703337200
- Primary Citation of Related Structures:
2PJL - PubMed Abstract:
Inverse agonists of the constitutively active human estrogen-related receptor alpha (ERRalpha, NR3B1) are of potential interest for several disease indications (e.g. breast cancer, metabolic diseases, or osteoporosis). ERRalpha is constitutively active, because its ligand binding pocket (LBP) is practically filled with side chains (in particular with Phe(328), which is replaced by Ala in ERRbeta and ERRgamma). We present here the crystal structure of the ligand binding domain of ERRalpha (containing the mutation C325S) in complex with the inverse agonist cyclohexylmethyl-(1-p-tolyl-1H-indol-3-ylmethyl)-amine (compound 1a), to a resolution of 2.3A(.) The structure reveals the dramatic multiple conformational changes in the LBP, which create the necessary space for the ligand. As a consequence of the new side chain conformation of Phe(328) (on helix H3), Phe(510)(H12) has to move away, and thus the activation helix H12 is displaced from its agonist position. This is a novel mechanism of H12 inactivation, different from ERRgamma, estrogen receptor (ER) alpha, and ERbeta. H12 binds (with a surprising binding mode) in the coactivator groove of its ligand binding domain, at a similar place as a coactivator peptide. This is in contrast to ERRgamma but resembles the situation for ERalpha (raloxifene or 4-hydroxytamoxifen complexes). Our results explain the novel molecular mechanism of an inverse agonist for ERRalpha and provide the basis for rational drug design to obtain isotype-specific inverse agonists of this potential new drug target. Despite a practically filled LBP, the finding that a suitable ligand can induce an opening of the cavity also has broad implications for other orphan nuclear hormone receptors (e.g. the NGFI-B subfamily).
Organizational Affiliation:
Novartis Institutes for BioMedical Research, CH-4002 Basel, Switzerland. Joerg.kallen@novartis.com