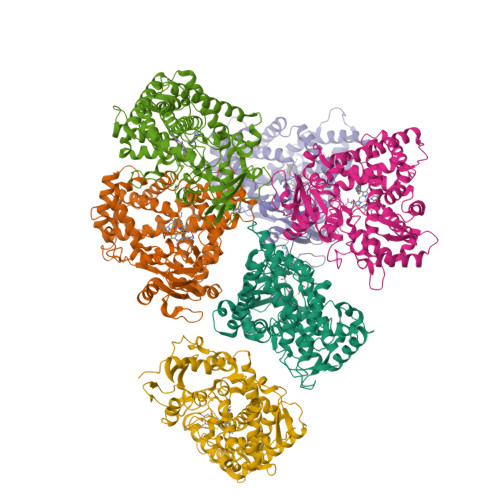
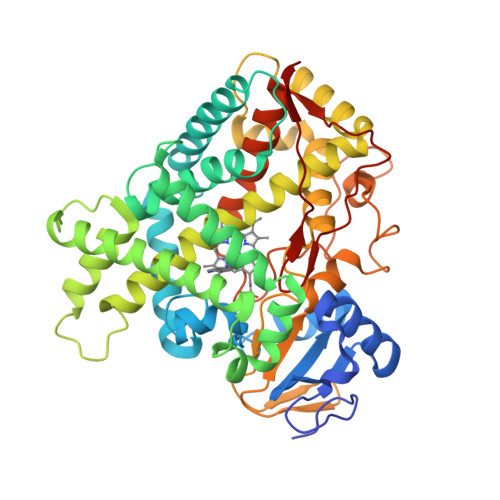
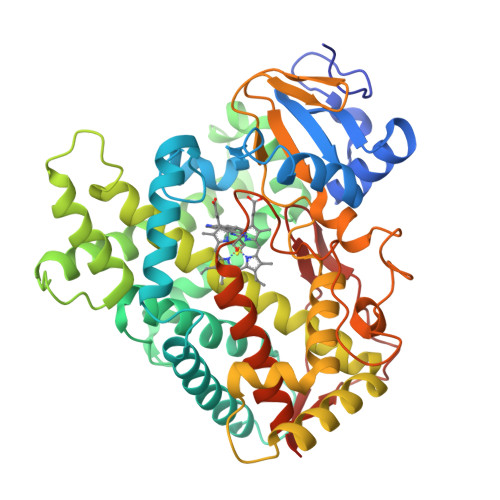
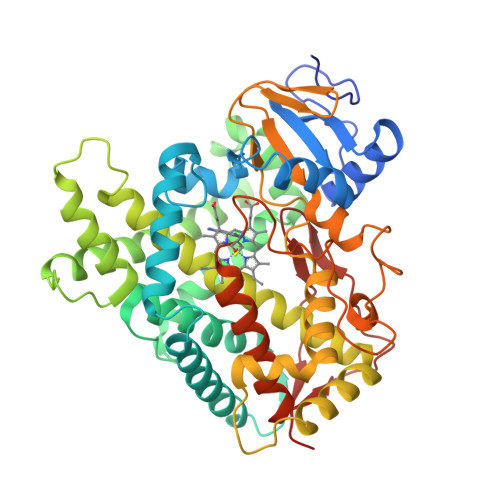
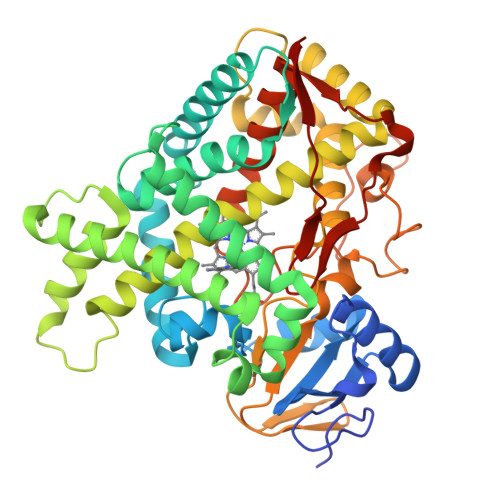
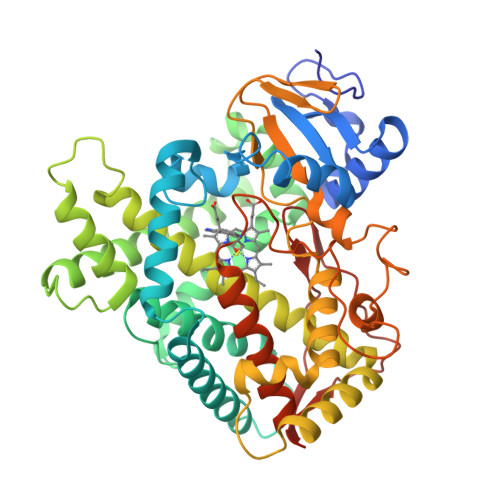
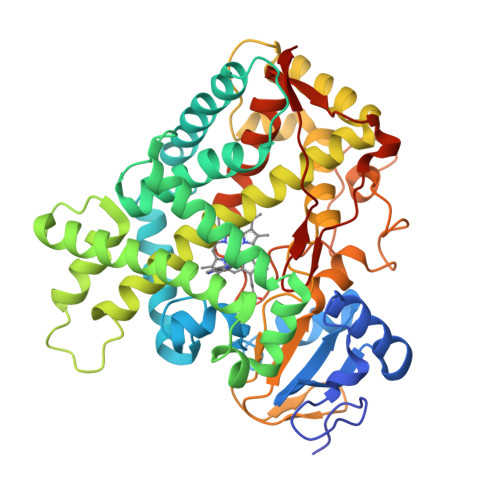
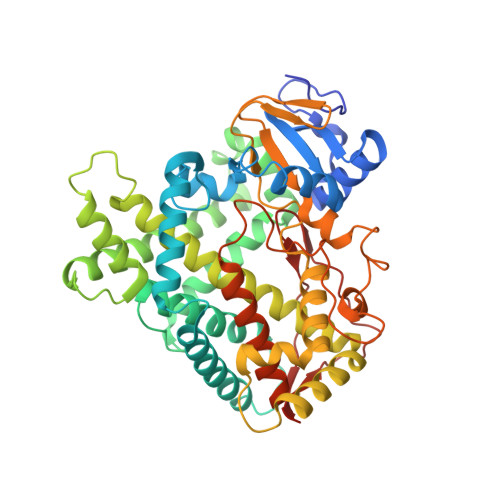
Structure of the human lung cytochrome P450 2A13.
Smith, B.D., Sanders, J.L., Porubsky, P.R., Lushington, G.H., Stout, C.D., Scott, E.E.(2007) J Biological Chem 282: 17306-17313
- PubMed: 17428784
- DOI: https://doi.org/10.1074/jbc.M702361200
- Primary Citation of Related Structures:
2P85 - PubMed Abstract:
The human lung cytochrome P450 2A13 (CYP2A13) activates the nicotine-derived procarcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) into DNA-altering compounds that cause lung cancer. Another cytochrome P450, CYP2A6, is also present in human lung, but at much lower levels. Although these two enzymes are 93.5% identical, CYP2A13 metabolizes NNK with much lower K(m) values than does CYP2A6. To investigate the structural differences between these two enzymes the structure of CYP2A13 was determined to 2.35A by x-ray crystallography and compared with structures of CYP2A6. As expected, the overall CYP2A13 and CYP2A6 structures are very similar with an average root mean square deviation of 0.5A for the Calpha atoms. Like CYP2A6, the CYP2A13 active site cavity is small and highly hydrophobic with a cluster of Phe residues composing the active site roof. Active site residue Asn(297) is positioned to hydrogen bond with an adventitious ligand, identified as indole. Amino acid differences between CYP2A6 and CYP2A13 at positions 117, 300, 301, and 208 relate to different orientations of the ligand plane in the two protein structures and may underlie the significant variations observed in binding and catalysis of many CYP2A ligands. In addition, docking studies suggest that residues 365 and 366 may also contribute to differences in NNK metabolism.
Organizational Affiliation:
Department of Medicinal Chemistry, University of Kansas, Lawrence, Kansas 66045, USA.