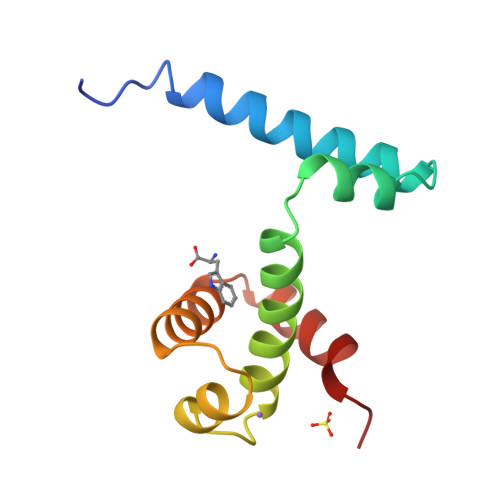
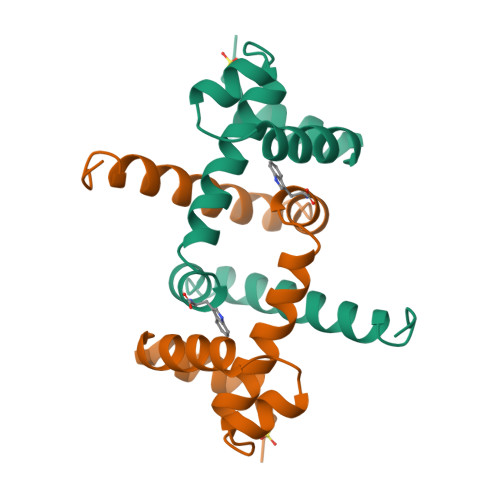
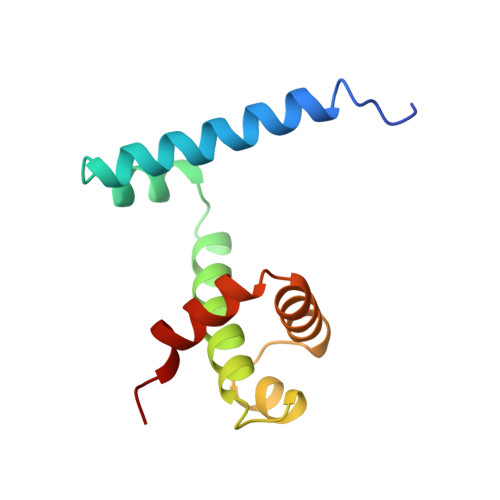
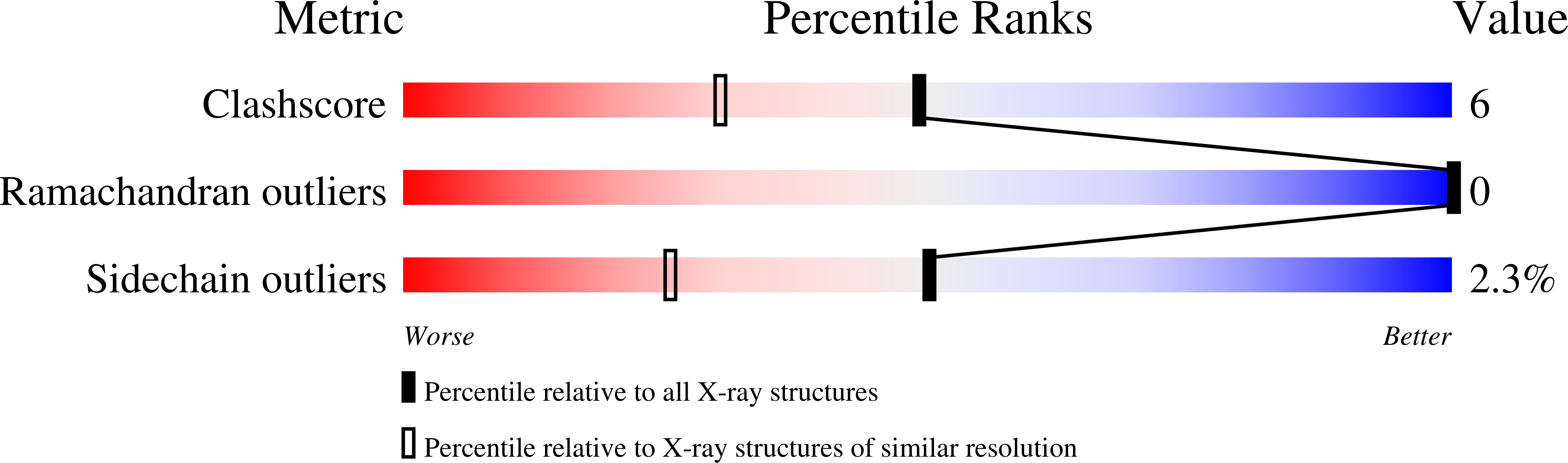Flexibility of the DNA-binding domains of trp repressor.
Lawson, C.L., Zhang, R.G., Schevitz, R.W., Otwinowski, Z., Joachimiak, A., Sigler, P.B.(1988) Proteins 3: 18-31
- PubMed: 3375234
- DOI: https://doi.org/10.1002/prot.340030103
- Primary Citation of Related Structures:
1WRP, 2OZ9, 3WRP - PubMed Abstract:
An orthorhombic crystal form of trp repressor (aporepressor plus L-tryptophan ligand) was solved by molecular replacement, refined to 1.65 A resolution, and compared to the structure of the repressor in trigonal crystals. Even though these two crystal forms of repressor were grown under identical conditions, the refined structures have distinctly different conformations of the DNA-binding domains. Unlike the repressor/aporepressor structural transition, the conformational shift is not caused by the binding or loss of the L-tryptophan ligand. We conclude that while L-tryptophan binding is essential for forming a specific complex with trp operator DNA, the corepressor ligand does not lock the repressor into a single conformation that is complementary to the operator. This flexibility may be required by the various binding modes proposed for trp repressor in its search for and adherence to its three different operator sites.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Chicago, Illinois 60637.