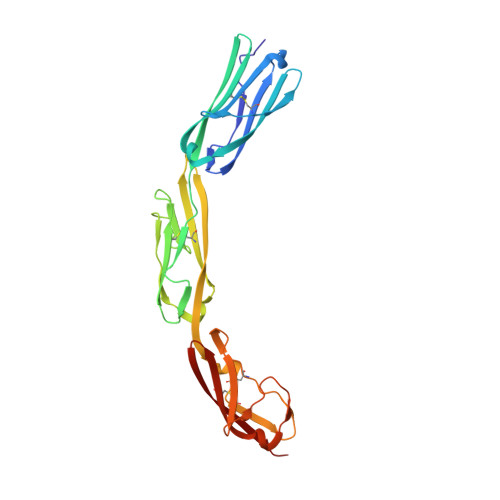
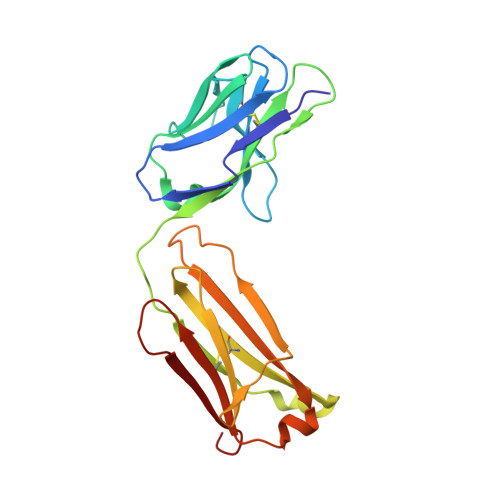
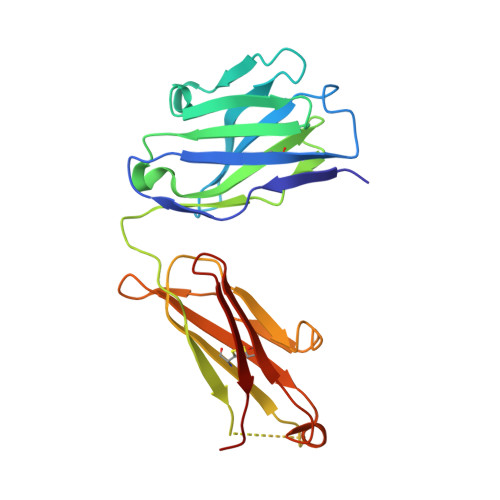
Structural plasticity in Ig superfamily domain 4 of ICAM-1 mediates cell surface dimerization.
Chen, X., Kim, T.D., Carman, C.V., Mi, L.Z., Song, G., Springer, T.A.(2007) Proc Natl Acad Sci U S A 104: 15358-15363
- PubMed: 17881562
- DOI: https://doi.org/10.1073/pnas.0707406104
- Primary Citation of Related Structures:
2OZ4 - PubMed Abstract:
The Ig superfamily (IgSF) intercellular adhesion molecule-1 (ICAM-1) equilibrates between monomeric and dimeric forms on the cell surface, and dimerization enhances cell adhesion. A crystal structure of ICAM-1 IgSF domains (D) 3-5 revealed a unique dimerization interface in which D4s of two protomers fuse through edge beta-strands to form a single super beta-sandwich domain. Here, we describe a crystal structure at 2.7-A resolution of monomeric ICAM-1 D3-D5, stabilized by the monomer-specific Fab CA7. CA7 binds to D5 in a region that is buried in the dimeric interface and is distal from the dimerization site in D4. In monomeric ICAM-1 D3-D5, a 16-residue loop in D4 that is disordered in the dimeric structure could clearly be traced as a BC loop, a short C strand, and a CE meander with a cis-Pro followed by a solvent-exposed, flexible four-residue region. Deletions of 6 or 10 residues showed that the C-strand is essential for monomer stability, whereas a distinct six-residue deletion showed little contribution of the CE meander. Mutation of two inward-pointing Leu residues in edge beta-strand E to Lys increased monomer stability, confirming the hypothesis that inward-pointing charged side chains on edge beta-strands are an important design feature to prevent beta-supersheet formation. Overall, the studies reveal that monomer-dimer transition is associated with a surprisingly large, physiologically relevant, IgSF domain rearrangement.
Organizational Affiliation:
Immune Disease Institute, Department of Pathology, Harvard Medical School, Boston, MA 02115, USA.