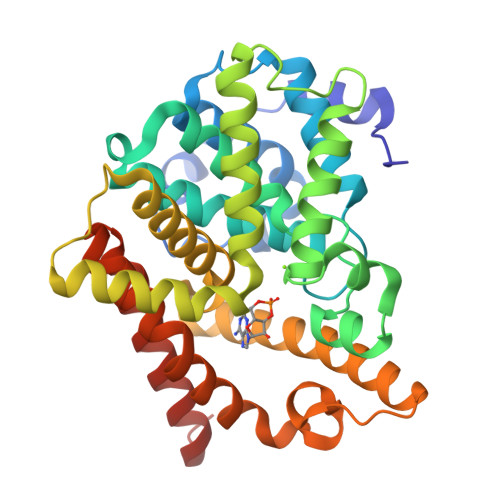
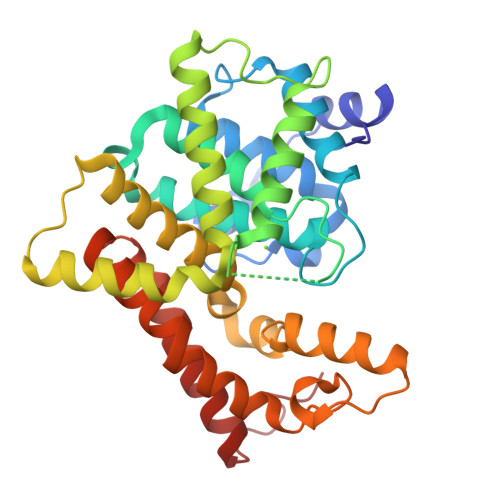
From the Cover: Structural insight into substrate specificity of phosphodiesterase 10.
Wang, H., Liu, Y., Hou, J., Zheng, M., Robinson, H., Ke, H.(2007) Proc Natl Acad Sci U S A 104: 5782-5787
- PubMed: 17389385
- DOI: https://doi.org/10.1073/pnas.0700279104
- Primary Citation of Related Structures:
2OUN, 2OUP, 2OUQ, 2OUR, 2OUS, 2OUU, 2OUV, 2OUY - PubMed Abstract:
Phosphodiesterases (PDEs) hydrolyze the second messengers cAMP and cGMP. It remains unknown how individual PDE families selectively recognize cAMP and cGMP. This work reports structural studies on substrate specificity. The crystal structures of the catalytic domains of the D674A and D564N mutants of PDE10A2 in complex with cAMP and cGMP reveal that two substrates bind to the active site with the same syn configuration but different orientations and interactions. The products AMP and GMP bind PDE10A2 with the anti configuration and interact with both divalent metals, in contrast to no direct contact of the substrates. The structures suggest that the syn configurations of cAMP and cGMP are the genuine substrates for PDE10 and the specificity is achieved through the different interactions and conformations of the substrates. The PDE10A2 structures also show that the conformation of the invariant glutamine is locked by two hydrogen bonds and is unlikely to switch for substrate recognition. Sequence alignment shows a potential pocket, in which variation of amino acids across PDE families defines the size and shape of the pocket and thus determines the substrate specificity.
Organizational Affiliation:
Department of Biochemistry and Biophysics and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC 27599-7260, USA.