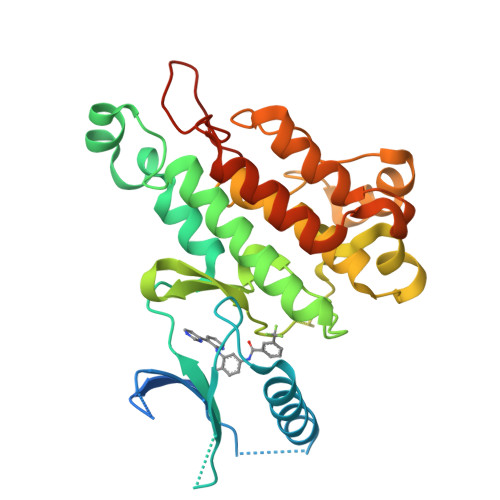
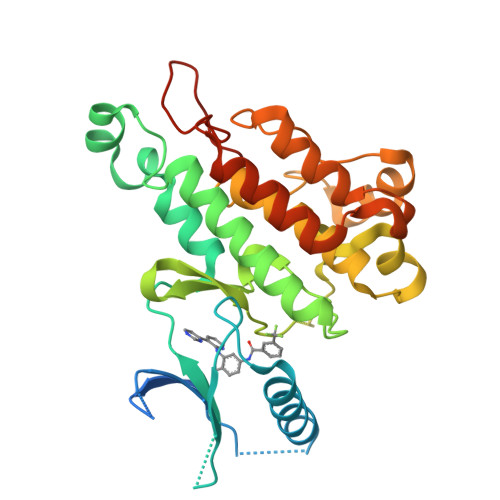
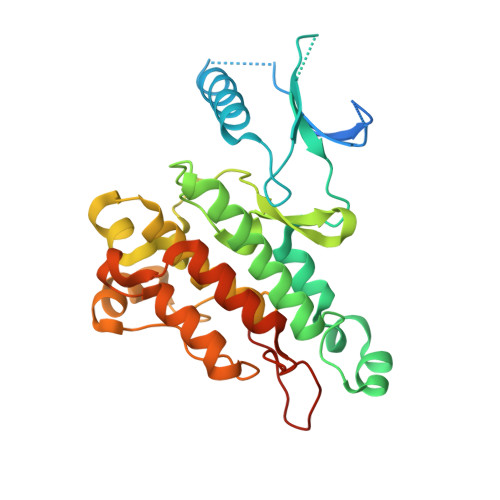
Synthesis, structural analysis, and SAR studies of triazine derivatives as potent, selective Tie-2 inhibitors.
Hodous, B.L., Geuns-Meyer, S.D., Hughes, P.E., Albrecht, B.K., Bellon, S., Caenepeel, S., Cee, V.J., Chaffee, S.C., Emery, M., Fretland, J., Gallant, P., Gu, Y., Johnson, R.E., Kim, J.L., Long, A.M., Morrison, M., Olivieri, P.R., Patel, V.F., Polverino, A., Rose, P., Wang, L., Zhao, H.(2007) Bioorg Med Chem Lett 17: 2886-2889
- PubMed: 17350837
- DOI: https://doi.org/10.1016/j.bmcl.2007.02.067
- Primary Citation of Related Structures:
2OO8, 2OSC - PubMed Abstract:
A novel class of selective Tie-2 inhibitors was derived from a multi-kinase inhibitor 1. By reversing the amide connectivity and incorporating aminotriazine or aminopyridine hinge-binding moieties, excellent Tie-2 potency and KDR selectivity could be achieved with 3-substituted terminal aryl rings. X-ray co-crystal structure analysis aided inhibitor design. This series was evaluated on the basis of potency, selectivity, and rat pharmacokinetic parameters.
Organizational Affiliation:
Department of Medicinal Chemistry, Amgen Inc., One Kendall Square, Building 1000, Cambridge, MA 02139, USA. bhodous@amgen.com