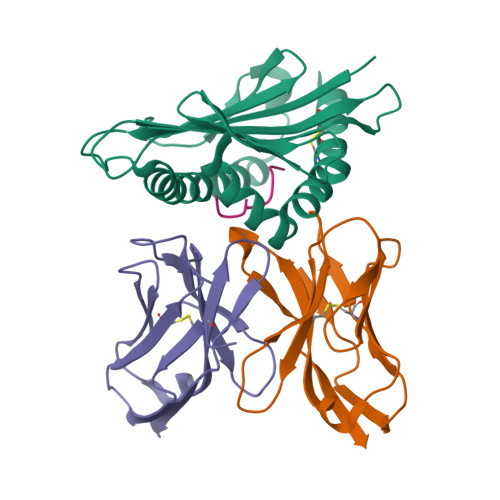
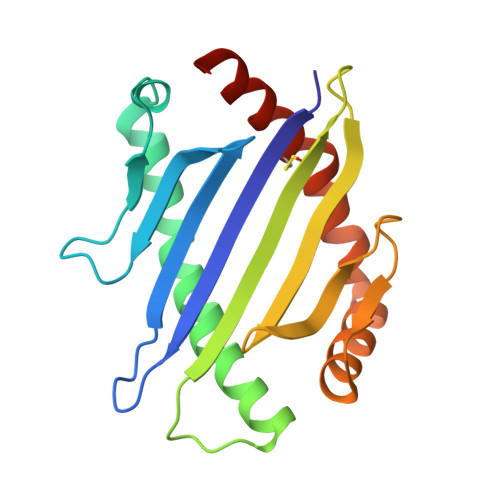
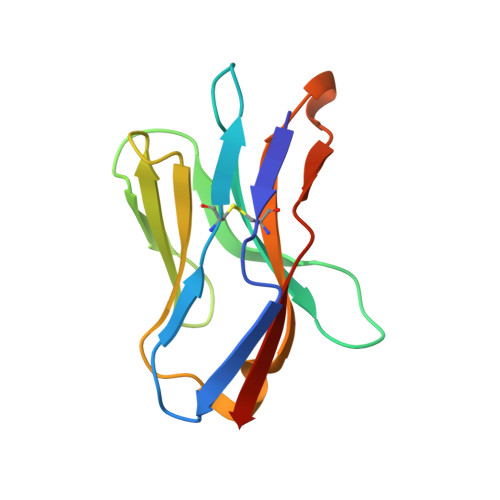
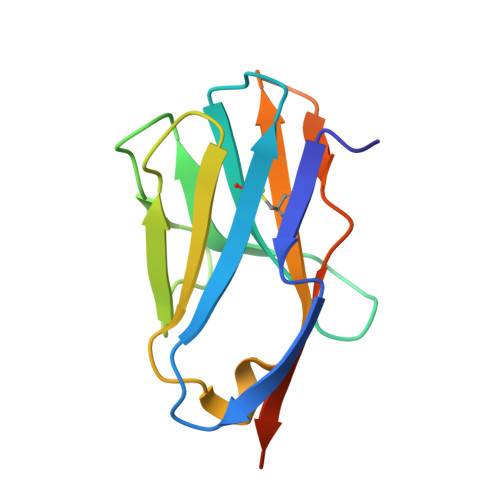
How a single T cell receptor recognizes both self and foreign MHC.
Colf, L.A., Bankovich, A.J., Hanick, N.A., Bowerman, N.A., Jones, L.L., Kranz, D.M., Garcia, K.C.(2007) Cell 129: 135-146
- PubMed: 17418792
- DOI: https://doi.org/10.1016/j.cell.2007.01.048
- Primary Citation of Related Structures:
2E7L, 2OI9 - PubMed Abstract:
alphabeta T cell receptors (TCRs) can crossreact with both self- and foreign- major histocompatibility complex (MHC) proteins in an enigmatic phenomenon termed alloreactivity. Here we present the 2.35 A structure of the 2C TCR complexed with its foreign ligand H-2L(d)-QL9. Surprisingly, we find that this TCR utilizes a different strategy to engage the foreign pMHC in comparison to the manner in which it recognizes a self ligand H-2K(b)-dEV8. 2C engages both shared and polymorphic residues on L(d) and K(b), as well as the unrelated QL9 and dEV8 peptide antigens, in unique pair-wise contacts, resulting in greater structural complementarity with the L(d)-QL9 complex. In the structure of an engineered, high-affinity 2C TCR variant bound to H-2L(d)-QL9, the "wild-type" TCR-MHC binding orientation persists despite modified TCR-CDR3alpha interactions with peptide. Thus, a single TCR recognizes two globally similar, but distinct ligands by divergent mechanisms, indicating that receptor-ligand crossreactivity can occur in the absence of molecular mimicry.
Organizational Affiliation:
Howard Hughes Medical Institute, Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA.