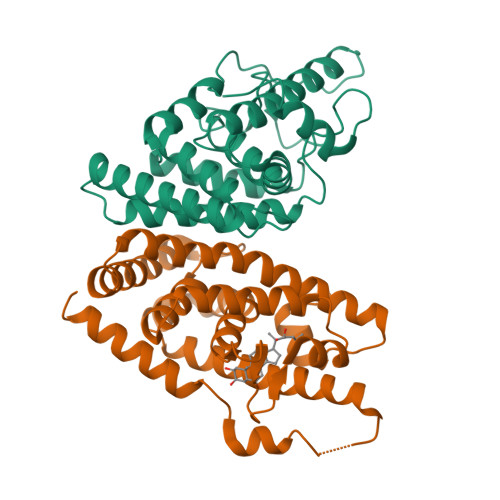
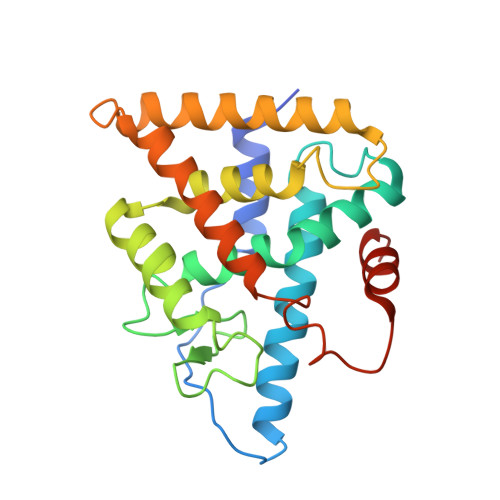
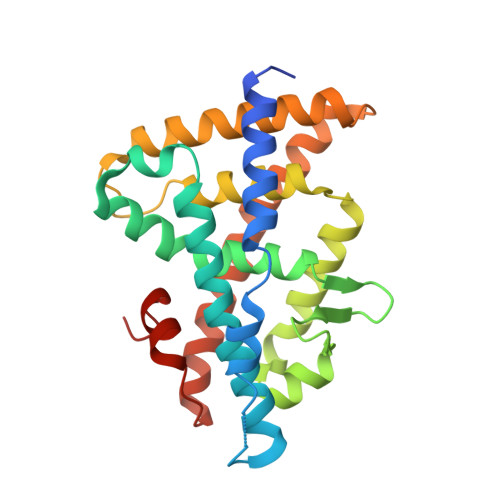
Structural and functional characterization of a novel type of ligand-independent RXR-USP receptor.
Iwema, T., Billas, I.M., Beck, Y., Bonneton, F., Nierengarten, H., Chaumot, A., Richards, G., Laudet, V., Moras, D.(2007) EMBO J 26: 3770-3782
- PubMed: 17673910
- DOI: https://doi.org/10.1038/sj.emboj.7601810
- Primary Citation of Related Structures:
2NXX - PubMed Abstract:
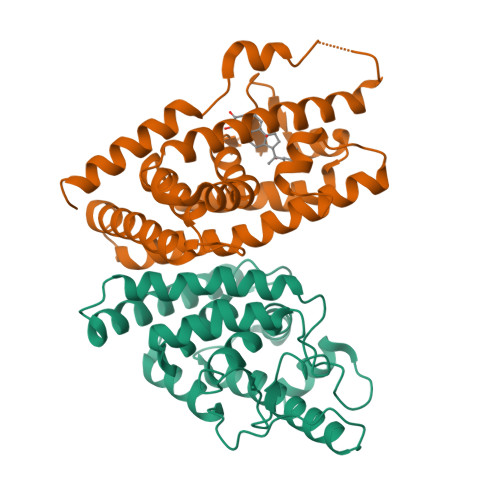
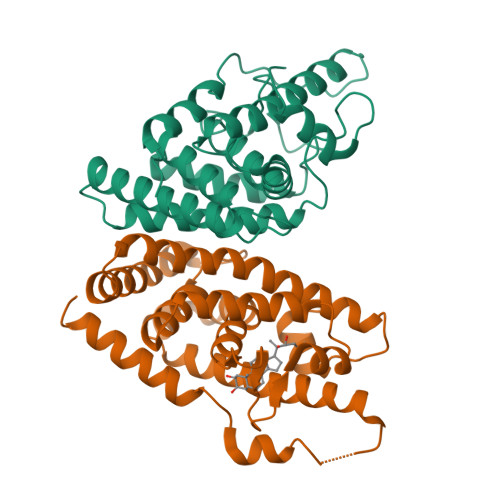
Retinoid X receptor (RXR) and Ultraspiracle (USP) play a central role as ubiquitous heterodimerization partners of many nuclear receptors. While it has long been accepted that a wide range of ligands can activate vertebrate/mollusc RXRs, the existence and necessity of specific endogenous ligands activating RXR-USP in vivo is still matter of intense debate. Here we report the existence of a novel type of RXR-USP with a ligand-independent functional conformation. Our studies involved Tribolium USP (TcUSP) as representative of most arthropod RXR-USPs, with high sequence homology to vertebrate/mollusc RXRs. The crystal structure of the ligand-binding domain of TcUSP was solved in the context of the functional heterodimer with the ecdysone receptor (EcR). While EcR exhibits a canonical ligand-bound conformation, USP adopts an original apo structure. Our functional data demonstrate that TcUSP is a constitutively silent partner of EcR, and that none of the RXR ligands can bind and activate TcUSP. These findings together with a phylogenetic analysis suggest that RXR-USPs have undergone remarkable functional shifts during evolution and give insight into receptor-ligand binding evolution and dynamics.
Organizational Affiliation:
IGBMC (Institut de Génétique et de Biologie Moléculaire et Cellulaire), UMR7104 CNRS, U596 INSERM, ULP, Département de Biologie et de Génomique Structurales, Illkirch, France.