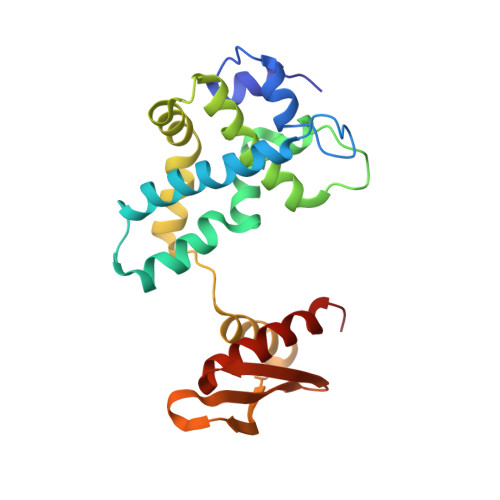
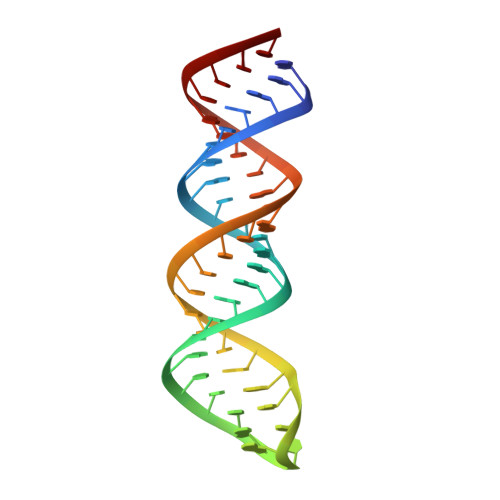
A stepwise model for double-stranded RNA processing by ribonuclease III.
Gan, J., Shaw, G., Tropea, J.E., Waugh, D.S., Court, D.L., Ji, X.(2007) Mol Microbiol 67: 143-154
- PubMed: 18047582
- DOI: https://doi.org/10.1111/j.1365-2958.2007.06032.x
- Primary Citation of Related Structures:
2NUE, 2NUF, 2NUG - PubMed Abstract:
RNA interference is mediated by small interfering RNAs produced by members of the ribonuclease III (RNase III) family represented by bacterial RNase III and eukaryotic Rnt1p, Drosha and Dicer. For mechanistic studies, bacterial RNase III has been a valuable model system for the family. Previously, we have shown that RNase III uses two catalytic sites to create the 2-nucleotide (nt) 3' overhangs in its products. Here, we present three crystal structures of RNase III in complex with double-stranded RNA, demonstrating how Mg(2+) is essential for the formation of a catalytically competent protein-RNA complex, how the use of two Mg(2+) ions can drive the hydrolysis of each phosphodiester bond, and how conformational changes in both the substrate and the protein are critical elements for assembling the catalytic complex. Moreover, we have modelled a protein-substrate complex and a protein-reaction intermediate (transition state) complex on the basis of the crystal structures. Together, the crystal structures and the models suggest a stepwise mechanism for RNase III to execute the phosphoryl transfer reaction.
Organizational Affiliation:
Center for Cancer Research, National Cancer Institute, National Institutes of Health, Frederick, MD 21702, USA.