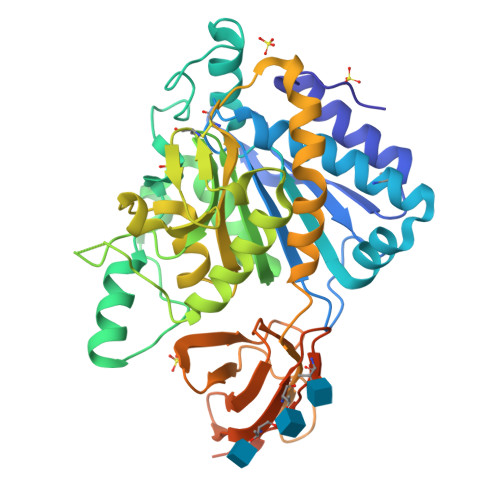
Crystal structure of the human carboxypeptidase N (kininase I) catalytic domain
Keil, C., Maskos, K., Than, M., Hoopes, J.T., Huber, R., Tan, F., Deddish, P.A., Erdoes, E.G., Skidgel, R.A., Bode, W.(2007) J Mol Biology 366: 504-516
- PubMed: 17157876
- DOI: https://doi.org/10.1016/j.jmb.2006.11.025
- Primary Citation of Related Structures:
2NSM - PubMed Abstract:
Human carboxypeptidase N (CPN), a member of the CPN/E subfamily of "regulatory" metallo-carboxypeptidases, is an extracellular glycoprotein synthesized in the liver and secreted into the blood, where it controls the activity of vasoactive peptide hormones, growth factors and cytokines by specifically removing C-terminal basic residues. Normally, CPN circulates in blood plasma as a hetero-tetramer consisting of two 83 kDa (CPN2) domains each flanked by a 48 to 55 kDa catalytic (CPN1) domain. We have prepared and crystallized the recombinant C-terminally truncated catalytic domain of human CPN1, and have determined and refined its 2.1 A crystal structure. The structural analysis reveals that CPN1 has a pear-like shape, consisting of a 319 residue N-terminal catalytic domain and an abutting, cylindrically shaped 79 residue C-terminal beta-sandwich transthyretin (TT) domain, more resembling CPD-2 than CPM. Like these other CPN/E members, two surface loops surrounding the active-site groove restrict access to the catalytic center, offering an explanation for why some larger protein carboxypeptidase inhibitors do not inhibit CPN. Modeling of the Pro-Phe-Arg C-terminal end of the natural substrate bradykinin into the active site shows that the S1' pocket of CPN1 might better accommodate P1'-Lys than Arg residues, in agreement with CPN's preference for cleaving off C-terminal Lys residues. Three Thr residues at the distal TT edge of CPN1 are O-linked to N-acetyl glucosamine sugars; equivalent sites in the membrane-anchored CPM are occupied by basic residues probably involved in membrane interaction. In tetrameric CPN, each CPN1 subunit might interact with the central leucine-rich repeat tandem of the cognate CPN2 subunit via a unique hydrophobic surface patch wrapping around the catalytic domain-TT interface, exposing the two active centers.
Organizational Affiliation:
Arbeitsgruppe Proteinaseforschung, Max-Planck-Institut für Biochemie, Am Klopferspitz 18, D-82152 Planegg-Martinsried, Germany.