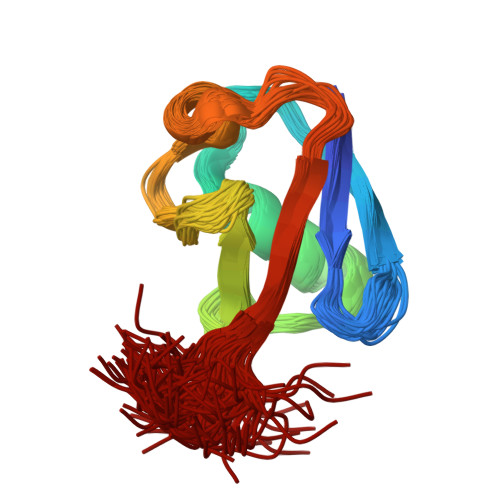
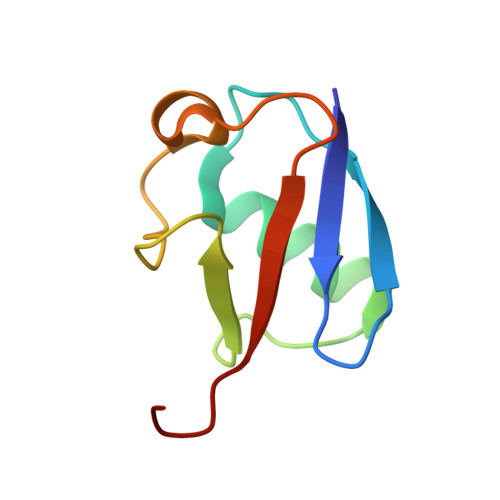
The MUMO (minimal under-restraining minimal over-restraining) method for the determination of native state ensembles of proteins
Richter, B., Gsponer, J., Varnai, P., Salvatella, X., Vendruscolo, M.(2007) J Biomol NMR 37: 117-135
- PubMed: 17225069
- DOI: https://doi.org/10.1007/s10858-006-9117-7
- Primary Citation of Related Structures:
2NR2 - PubMed Abstract:
While reliable procedures for determining the conformations of proteins are available, methods for generating ensembles of structures that also reflect their flexibility are much less well established. Here we present a systematic assessment of the ability of ensemble-averaged molecular dynamics simulations with ensemble-averaged NMR restraints to simultaneously reproduce the average structure of proteins and their associated dynamics. We discuss the effects that under-restraining (overfitting) and over-restraining (underfitting) have on the structures generated in ensemble-averaged molecular simulations. We then introduce the MUMO (minimal under-restraining minimal over-restraining) method, a procedure in which different observables are averaged over a different number of molecules. As both over-restraining and under-restraining are significantly reduced in the MUMO method, it is possible to generate ensembles of conformations that accurately characterize both the structure and the dynamics of native states of proteins. The application of the MUMO method to the protein ubiquitin yields a high-resolution structural ensemble with an RDC Q-factor of 0.19.
Organizational Affiliation:
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK.