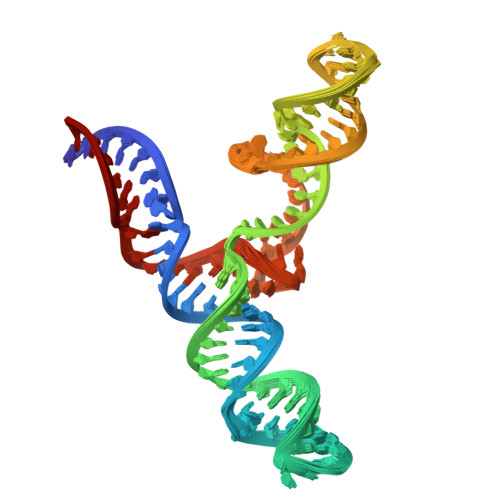
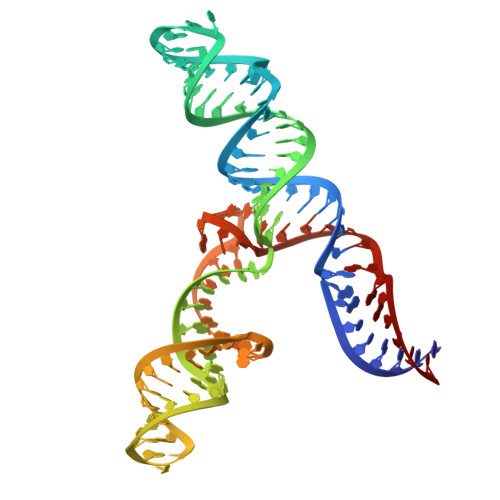
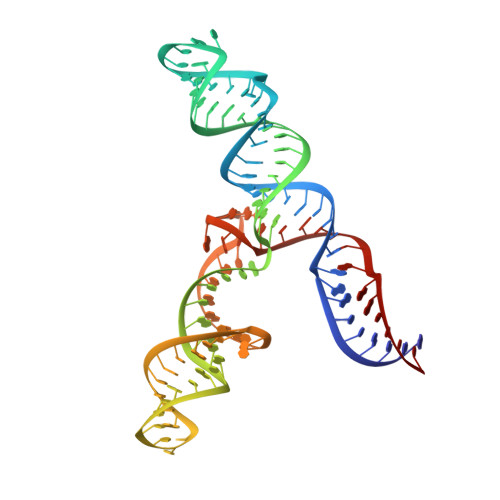
An accurately preorganized IRES RNA structure enables eIF4G capture for initiation of viral translation.
Imai, S., Kumar, P., Hellen, C.U., D'Souza, V.M., Wagner, G.(2016) Nat Struct Mol Biol 23: 859-864
- PubMed: 27525590
- DOI: https://doi.org/10.1038/nsmb.3280
- Primary Citation of Related Structures:
2NBX, 2NBY, 2NBZ, 2NC0, 2NC1 - PubMed Abstract:
Many viruses bypass canonical cap-dependent translation in host cells by using internal ribosomal entry sites (IRESs) in their transcripts; IRESs hijack initiation factors for the assembly of initiation complexes. However, it is currently unknown how IRES RNAs recognize initiation factors that have no endogenous RNA binding partners; in a prominent example, the IRES of encephalomyocarditis virus (EMCV) interacts with the HEAT-1 domain of eukaryotic initiation factor 4G (eIF4G). Here we report the solution structure of the J-K region of this IRES and show that its stems are precisely organized to position protein-recognition bulges. This multisite interaction mechanism operates on an all-or-nothing principle in which all domains are required. This preorganization is accomplished by an 'adjuster module': a pentaloop motif that acts as a dual-sided docking station for base-pair receptors. Because subtle changes in the orientation abrogate protein capture, our study highlights how a viral RNA acquires affinity for a target protein.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts, USA.