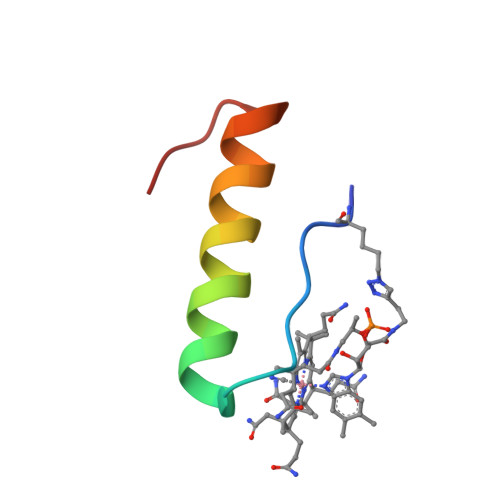
Solution Structure and Constrained Molecular Dynamics Study of Vitamin B12 Conjugates of the Anorectic Peptide PYY(3-36).
Henry, K.E., Kerwood, D.J., Allis, D.G., Workinger, J.L., Bonaccorso, R.L., Holz, G.G., Roth, C.L., Zubieta, J., Doyle, R.P.(2016) ChemMedChem 11: 1015-1021
- PubMed: 27027248
- DOI: https://doi.org/10.1002/cmdc.201600073
- Primary Citation of Related Structures:
2NA5 - PubMed Abstract:
Vitamin B12 -peptide conjugates have considerable therapeutic potential through improved pharmacokinetic and/or pharmacodynamic properties imparted on the peptide upon covalent attachment to vitamin B12 (B12 ). There remains a lack of structural studies investigating the effects of B12 conjugation on peptide secondary structure. Determining the solution structure of a B12 -peptide conjugate or conjugates and measuring functions of the conjugate(s) at the target peptide receptor may offer considerable insight concerning the future design of fully optimized conjugates. This methodology is especially useful in tandem with constrained molecular dynamics (MD) studies, such that predictions may be made about conjugates not yet synthesized. Focusing on two B12 conjugates of the anorectic peptide PYY(3-36), one of which was previously demonstrated to have improved food intake reduction compared with PYY(3-36), we performed NMR structural analyses and used the information to conduct MD simulations. The study provides rare structural insight into vitamin B12 conjugates and validates the fact that B12 can be conjugated to a peptide without markedly affecting peptide secondary structure.
Organizational Affiliation:
Department of Chemistry, Center for Science and Technology, Syracuse University, 111 College Place, Syracuse, NY, 13244, USA.