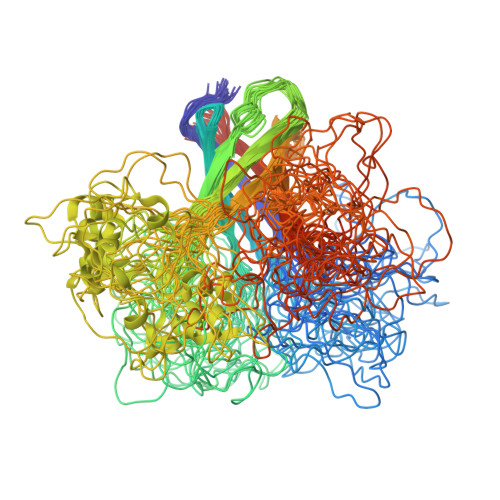
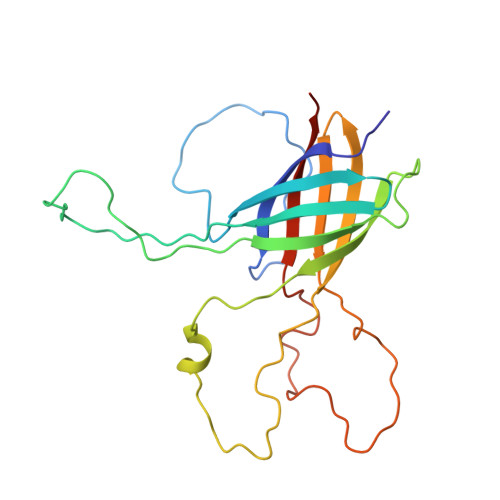
OprG Harnesses the Dynamics of its Extracellular Loops to Transport Small Amino Acids across the Outer Membrane of Pseudomonas aeruginosa.
Kucharska, I., Seelheim, P., Edrington, T., Liang, B., Tamm, L.K.(2015) Structure 23: 2234-2245
- PubMed: 26655471
- DOI: https://doi.org/10.1016/j.str.2015.10.009
- Primary Citation of Related Structures:
2N6L, 2N6P - PubMed Abstract:
OprG is an outer membrane protein of Pseudomonas aeruginosa whose function as an antibiotic-sensitive porin has been controversial and not well defined. Circumstantial evidence led to the proposal that OprG might transport hydrophobic compounds by using a lateral gate in the barrel wall thought to be lined by three conserved prolines. To test this hypothesis and to find the physiological substrates of OprG, we reconstituted the purified protein into liposomes and found it to facilitate the transport of small amino acids such as glycine, alanine, valine, and serine, which was confirmed by Pseudomonas growth assays. The structures of wild-type and a critical proline mutant were determined by nuclear magnetic resonance in dihexanoyl-phosphatidylcholine micellar solutions. Both proteins formed eight-stranded β-barrels with flexible extracellular loops. The interfacial prolines did not form a lateral gate in these structures, but loop 3 exhibited restricted motions in the inactive P92A mutant but not in wild-type OprG.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, Center for Membrane Biology, University of Virginia, Charlottesville, VA 22908, USA.