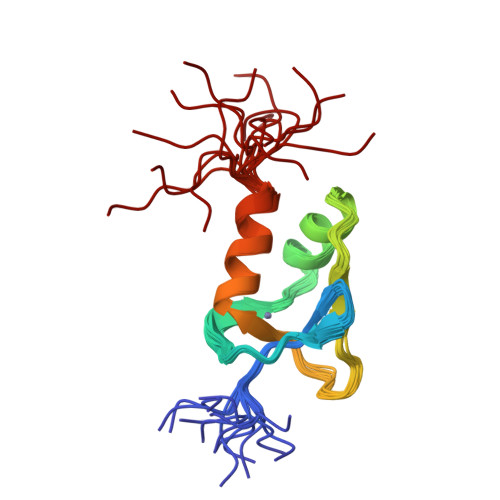
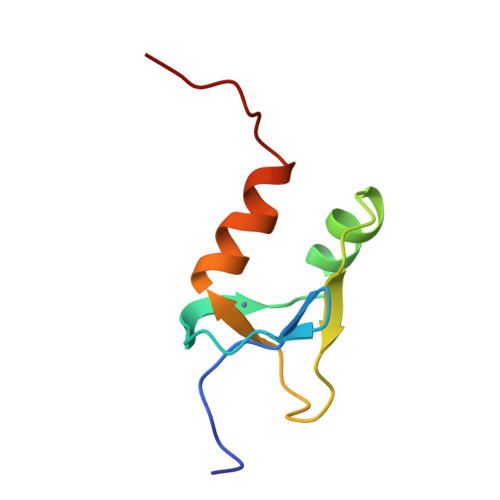
Redox-dependent disulfide bond formation in SAP30L corepressor protein: Implications for structure and function.
Laitaoja, M., Tossavainen, H., Pihlajamaa, T., Valjakka, J., Viiri, K., Lohi, O., Permi, P., Janis, J.(2016) Protein Sci 25: 572-586
- PubMed: 26609676
- DOI: https://doi.org/10.1002/pro.2849
- Primary Citation of Related Structures:
2N1U - PubMed Abstract:
Sin3A-associated protein 30-like (SAP30L) is one of the key proteins in a multi-subunit protein complex involved in transcriptional regulation via histone deacetylation. SAP30L, together with a highly homologous SAP30 as well as other SAP proteins (i.e., SAP25, SAP45, SAP130, and SAP180), is an essential component of the Sin3A corepressor complex, although its actual role has remained elusive. SAP30L is thought to function as an important stabilizing and bridging molecule in the complex and to mediate its interactions with other corepressors. SAP30L has been previously shown to contain an N-terminal Cys3 His type zinc finger (ZnF) motif, which is responsible for the key protein-protein, protein-DNA, and protein-lipid interactions. By using high-resolution mass spectrometry, we studied a redox-dependent disulfide bond formation in SAP30L ZnF as a regulatory mechanism for its structure and function. We showed that upon oxidative stress SAP30L undergoes the formation of two specific disulfide bonds, a vicinal Cys29-Cys30 and Cys38-Cys74, with a concomitant release of the coordinated zinc ion. The oxidized protein was shown to remain folded in solution and to bind signaling phospholipids. We also determined a solution NMR structure for SAP30L ZnF that showed an overall fold similar to that of SAP30, determined earlier. The NMR titration experiments with lipids and DNA showed that the binding is mediated by the C-terminal tail as well as both α-helices of SAP30L ZnF. The implications of these results for the structure and function of SAP30L are discussed.
Organizational Affiliation:
Department of Chemistry, University of Eastern Finland, Joensuu, Finland.