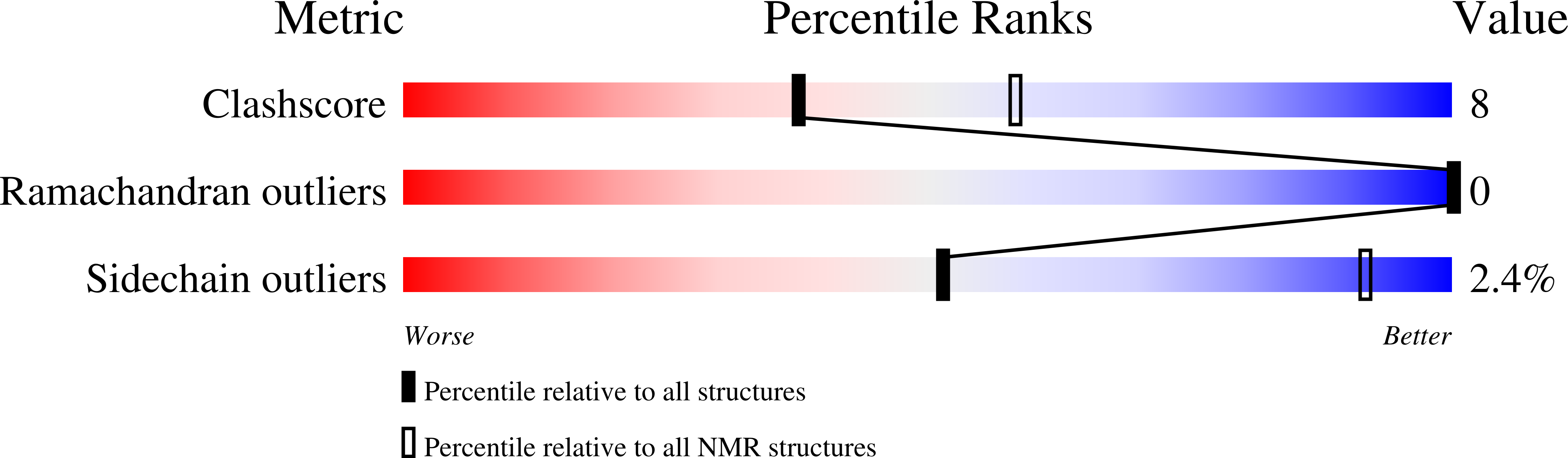Molecular Basis for Impaired DNA Damage Response Function Associated with the RAP80 Delta E81 Defect.
Anamika, Markin, C.J., Rout, M.K., Spyracopoulos, L.(2014) J Biological Chem 289: 12852-12862
- PubMed: 24627472
- DOI: https://doi.org/10.1074/jbc.M113.538280
- Primary Citation of Related Structures:
2MKF, 2MKG - PubMed Abstract:
Signal transduction within the DNA damage response is driven by the flux of protein-protein interaction cascades that ultimately recruit repair complexes to sites of damage. The protein RAP80 plays a central role in the damage response by targeting BRCA1/BRCA2 tumor suppressors to DNA damage foci through multivalent binding of Lys-63-linked polyubiquitin chains. Mutations within the high penetrance BRCA1/BRCA2 genes account for ∼20% of familial breast cancers. The genetic basis for the remaining cancers remains unknown, but may involve defects in binding partners for BRCA1 and BRCA2 that lead to impaired targeting to foci and a concomitant role in the pathogenesis of cancer. Recently, an in-frame deletion mutation (ΔE81) in a conserved region from the first ubiquitin interaction motif of RAP80 has been linked to an increase in chromosomal abnormalities. Using NMR spectroscopy, we demonstrate that the N-cap motif within the α-helix of the first ubiquitin interaction motif from ΔE81 undergoes a structural frameshift that leads to abolishment of multivalent binding of polyubiquitin chains. Loss of this single glutamate residue disrupts favorable electrostatic interactions between RAP80 and ubiquitin, establishing a plausible molecular basis for a potential predisposition to cancer unrelated to mutations within BRCA1/BRCA2 genes.
Organizational Affiliation:
From the Department of Biochemistry, University of Alberta, Edmonton, Alberta T6G 2H7, Canada.