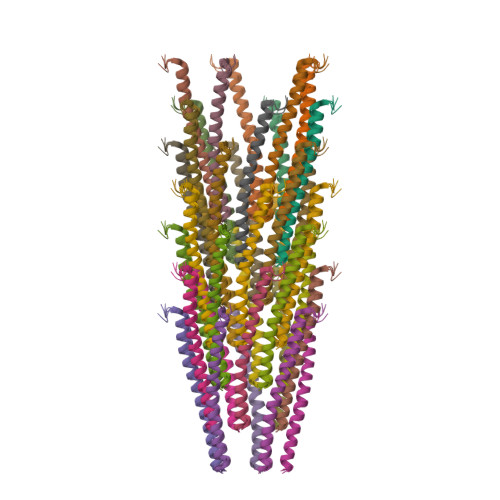The NMR-Rosetta capsid model of M13 bacteriophage reveals a quadrupled hydrophobic packing epitope.
Morag, O., Sgourakis, N.G., Baker, D., Goldbourt, A.(2015) Proc Natl Acad Sci U S A 112: 971-976
- PubMed: 25587134
- DOI: https://doi.org/10.1073/pnas.1415393112
- Primary Citation of Related Structures:
2MJZ - PubMed Abstract:
Filamentous phage are elongated semiflexible ssDNA viruses that infect bacteria. The M13 phage, belonging to the family inoviridae, has a length of ∼1 μm and a diameter of ∼7 nm. Here we present a structural model for the capsid of intact M13 bacteriophage using Rosetta model building guided by structure restraints obtained from magic-angle spinning solid-state NMR experimental data. The C5 subunit symmetry observed in fiber diffraction studies was enforced during model building. The structure consists of stacked pentamers with largely alpha helical subunits containing an N-terminal type II β-turn; there is a rise of 16.6-16.7 Å and a tilt of 36.1-36.6° between consecutive pentamers. The packing of the subunits is stabilized by a repeating hydrophobic stacking pocket; each subunit participates in four pockets by contributing different hydrophobic residues, which are spread along the subunit sequence. Our study provides, to our knowledge, the first magic-angle spinning NMR structure of an intact filamentous virus capsid and further demonstrates the strength of this technique as a method of choice to study noncrystalline, high-molecular-weight molecular assemblies.
Organizational Affiliation:
School of Chemistry, Raymond and Beverly Sackler Faculty of Exact Sciences, Tel Aviv University, Ramat Aviv 69978, Tel Aviv, Israel; and.