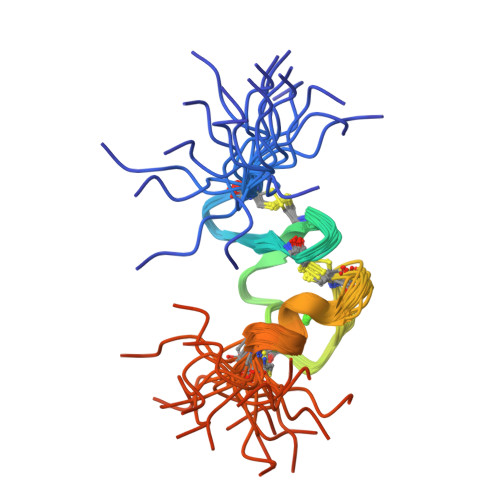
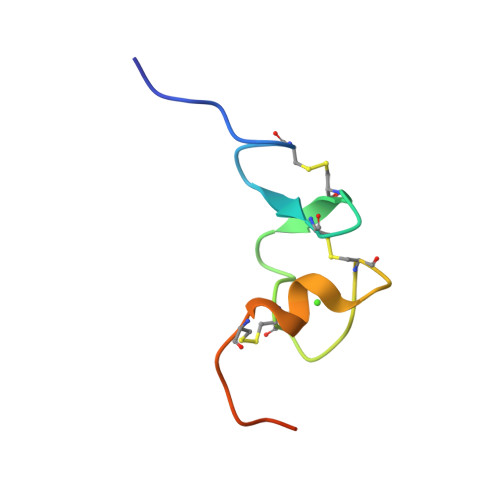
Gentamicin binds to the megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: insight from the nmr structure of the 10th complement type repeat domain alone and in complex with gentamicin.
Dagil, R., O'Shea, C., Nykjar, A., Bonvin, A.M., Kragelund, B.B.(2013) J Biological Chem 288: 4424-4435
- PubMed: 23275343
- DOI: https://doi.org/10.1074/jbc.M112.434159
- Primary Citation of Related Structures:
2M0P - PubMed Abstract:
Gentamicin is an aminoglycoside widely used in treatments of, in particular, enterococcal, mycobacterial, and severe Gram-negative bacterial infections. Large doses of gentamicin cause nephrotoxicity and ototoxicity, entering the cell via the receptor megalin. Until now, no structural information has been available to describe the interaction with gentamicin in atomic detail, and neither have any three-dimensional structures of domains from the human megalin receptor been solved. To address this gap in our knowledge, we have solved the NMR structure of the 10th complement type repeat of human megalin and investigated its interaction with gentamicin. Using NMR titration data in HADDOCK, we have generated a three-dimensional model describing the complex between megalin and gentamicin. Gentamicin binds to megalin with low affinity and exploits the common ligand binding motif previously described (Jensen, G. A., Andersen, O. M., Bonvin, A. M., Bjerrum-Bohr, I., Etzerodt, M., Thogersen, H. C., O'Shea, C., Poulsen, F. M., and Kragelund, B. B. (2006) J. Mol. Biol. 362, 700-716) utilizing the indole side chain of Trp-1126 and the negatively charged residues Asp-1129, Asp-1131, and Asp-1133. Binding to megalin is highly similar to gentamicin binding to calreticulin. We discuss the impact of this novel insight for the future structure-based design of gentamicin antagonists.
Organizational Affiliation:
Structural Biology and NMR Laboratory, Department of Biology, University of Copenhagen, Ole Maaloes Vej 5, DK-2200 Copenhagen, Denmark.