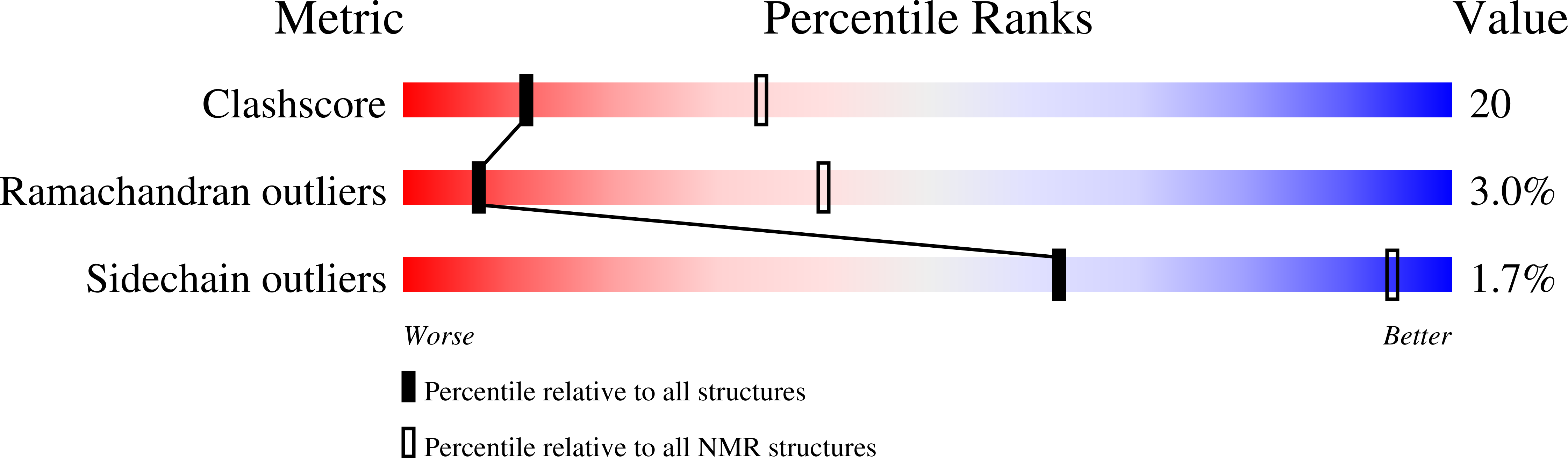Structure of the Plasmodium 6-cysteine s48/45 domain.
Arredondo, S.A., Cai, M., Takayama, Y., Macdonald, N.J., Anderson, D.E., Aravind, L., Clore, G.M., Miller, L.H.(2012) Proc Natl Acad Sci U S A 109: 6692-6697
- PubMed: 22493233
- DOI: https://doi.org/10.1073/pnas.1204363109
- Primary Citation of Related Structures:
2LOE - PubMed Abstract:
The s48/45 domain was first noted in Plasmodium proteins more than 15 y ago. Previously believed to be unique to Plasmodium, the s48/45 domain is present in other aconoidasidans. In Plasmodium, members of the s48/45 family of proteins are localized on the surface of the parasite in different stages, mostly by glycosylphosphatydylinositol-anchoring. Members such as P52 and P36 seem to play a role in invasion of hepatocytes, and Pfs230 and Pfs48/45 are involved in fertilization in the sexual stages and have been consistently studied as targets of transmission-blocking vaccines for years. In this report, we present the molecular structure for the s48/45 domain corresponding to the C-terminal domain of the blood-stage protein Pf12 from Plasmodium falciparum, obtained by NMR. Our results indicate that this domain is a β-sandwich formed by two sheets with a mixture of parallel and antiparallel strands. Of the six conserved cysteines, two pairs link the β-sheets by two disulfide bonds, and the third pair forms a bond outside the core. The structure of the s48/45 domain conforms well to the previously defined surface antigen 1 (SAG1)-related-sequence (SRS) fold observed in the SAG family of surface antigens found in Toxoplasma gondii. Despite extreme sequence divergence, remarkable spatial conservation of one of the disulfide bonds is observed, supporting the hypothesis that the domains have evolved from a common ancestor. Furthermore, a homologous domain is present in ephrins, raising the possibility that the precursor of the s48/45 and SRS domains emerged from an ancient transfer to Apicomplexa from metazoan hosts.
Organizational Affiliation:
Laboratory of Immunogenetics, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD 20852, USA.