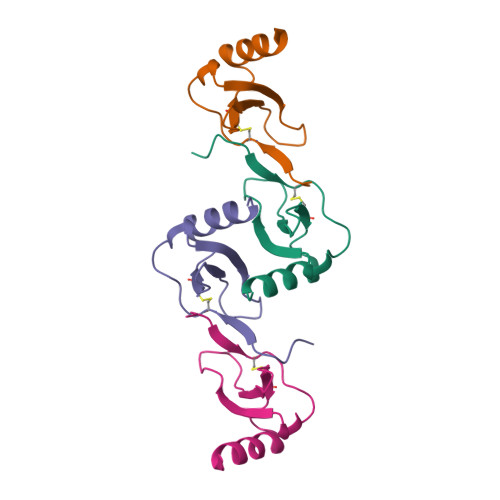
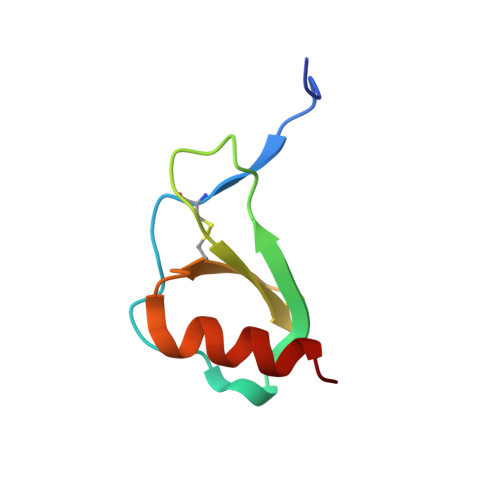
Oligomeric Structure of the Chemokine CCL5/RANTES from NMR, MS, and SAXS Data.
Wang, X., Watson, C., Sharp, J.S., Handel, T.M., Prestegard, J.H.(2011) Structure 19: 1138-1148
- PubMed: 21827949
- DOI: https://doi.org/10.1016/j.str.2011.06.001
- Primary Citation of Related Structures:
2L9H - PubMed Abstract:
CCL5 (RANTES) is a proinflammatory chemokine known to activate leukocytes through its receptor, CCR5. Although the monomeric form of CCL5 is sufficient to cause cell migration in vitro, CCL5's propensity for aggregation is essential for migration in vivo, T cell activation and apoptosis, and HIV entry into cells. However, there is currently no structural information on CCL5 oligomers larger than the canonical CC chemokine dimer. In this study the solution structure of a CCL5 oligomer was investigated using an integrated approach, including NMR residual dipolar couplings to determine allowed relative orientations of the component monomers, SAXS to restrict overall shape, and hydroxyl radical footprinting and NMR cross-saturation experiments to identify interface residues. The resulting model of the CCL5 oligomer provides a basis for explaining the disaggregating effect of E66 and E26 mutations and suggests mechanisms by which glycosaminoglycan binding may promote oligomer formation and facilitate cell migration in vivo.
Organizational Affiliation:
Complex Carbohydrate Research Center, University of Georgia, Athens, GA 30602, USA.