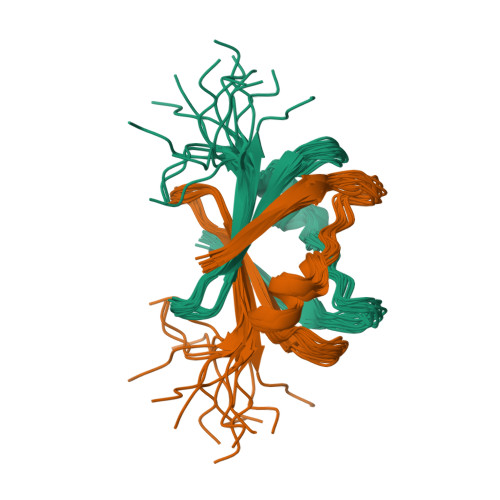
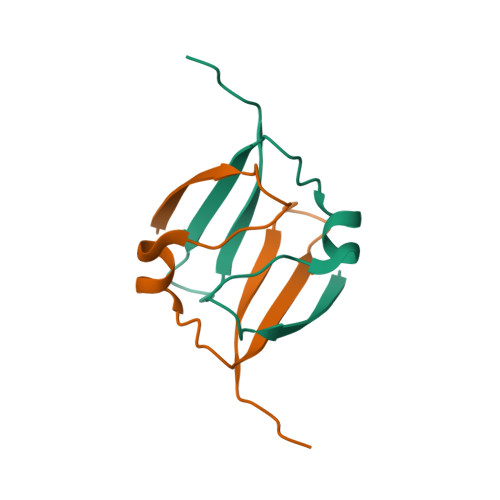
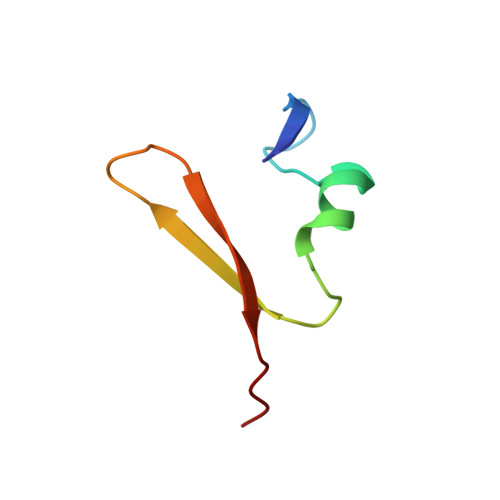
The DNA-recognition fold of Sso7c4 suggests a new member of SpoVT-AbrB superfamily from archaea.
Hsu, C.-H., Wang, A.H.-J.(2011) Nucleic Acids Res 39: 6764-6774
- PubMed: 21546550
- DOI: https://doi.org/10.1093/nar/gkr283
- Primary Citation of Related Structures:
2L66 - PubMed Abstract:
Organisms growing at elevated temperatures face the challenge of maintaining the integrity of their genetic materials. Archaea possess unique chromatin proteins for gene organization and information processing. We present the solution structure of Sso7c4 from Sulfolobus solfataricus, which has a homodimeric DNA-binding fold forming a swapped β-loop-β 'Tai-Chi' topology. The fold is reminiscent of the N-terminal DNA-binding domain of AbrB and MazE. In addition, several amide resonances in the heteronuclear single quantum coherence spectra of Sso7c4 are shifted and broadened with the addition of small amounts of duplex DNA oligomers. The locations of the corresponding amides in the Sso7c4 structure define its DNA-interacting surface. NMR spectra of DNA titrated with the protein further indicated that Sso7c4 interacts with DNA in the major groove. Taken together, a plausible model for the Sso7c4-DNA complex is presented, in which the DNA double helix is curved around the protein dimer.
Organizational Affiliation:
Department of Agricultural Chemistry, National Taiwan University, Taipei, Taiwan.