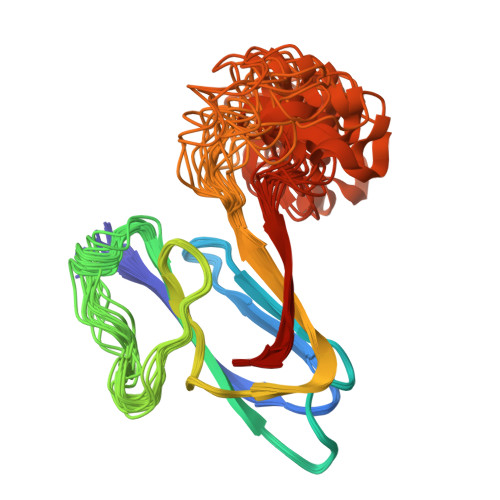
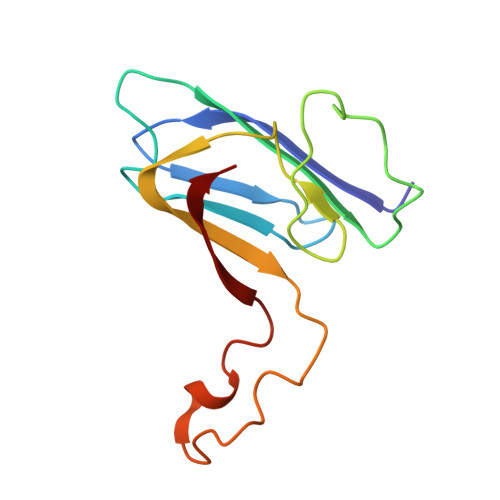
Cdc42-dependent formation of the ZO-1/MRCKb complex at the leading edge controls cell migration
Huo, L., Wen, W., Wang, R., Kam, C., Xia, J., Feng, W., Zhang, M.(2011) EMBO J 30: 665-678
- PubMed: 21240187
- DOI: https://doi.org/10.1038/emboj.2010.353
- Primary Citation of Related Structures:
2KXR, 2KXS - PubMed Abstract:
Zonula occludens (ZO)-1 is a multi-domain scaffold protein known to have critical roles in the establishment of cell-cell adhesions and the maintenance of stable tissue structures through the targeting, anchoring, and clustering of transmembrane adhesion molecules and cytoskeletal proteins. Here, we report that ZO-1 directly binds to MRCKβ, a Cdc42 effector kinase that modulates cell protrusion and migration, at the leading edge of migrating cells. Structural studies reveal that the binding of a β hairpin from GRINL1A converts ZO-1 ZU5 into a complete ZU5-fold. A similar interaction mode is likely to occur between ZO-1 ZU5 and MRCKβ. The interaction between ZO-1 and MRCKβ requires the kinase to be primed by Cdc42 due to the closed conformation of the kinase. Formation of the ZO-1/MRCKβ complex enriches the kinase at the lamellae of migrating cells. Disruption of the ZO-1/MRCKβ complex inhibits MRCKβ-mediated cell migration. These results demonstrate that ZO-1, a classical scaffold protein with accepted roles in maintaining cell-cell adhesions in stable tissues, also has an active role in cell migration during processes such as tissue development and remodelling.
Organizational Affiliation:
Division of Life Science, Molecular Neuroscience Center, State Key Laboratory of Molecular Neuroscience, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong.