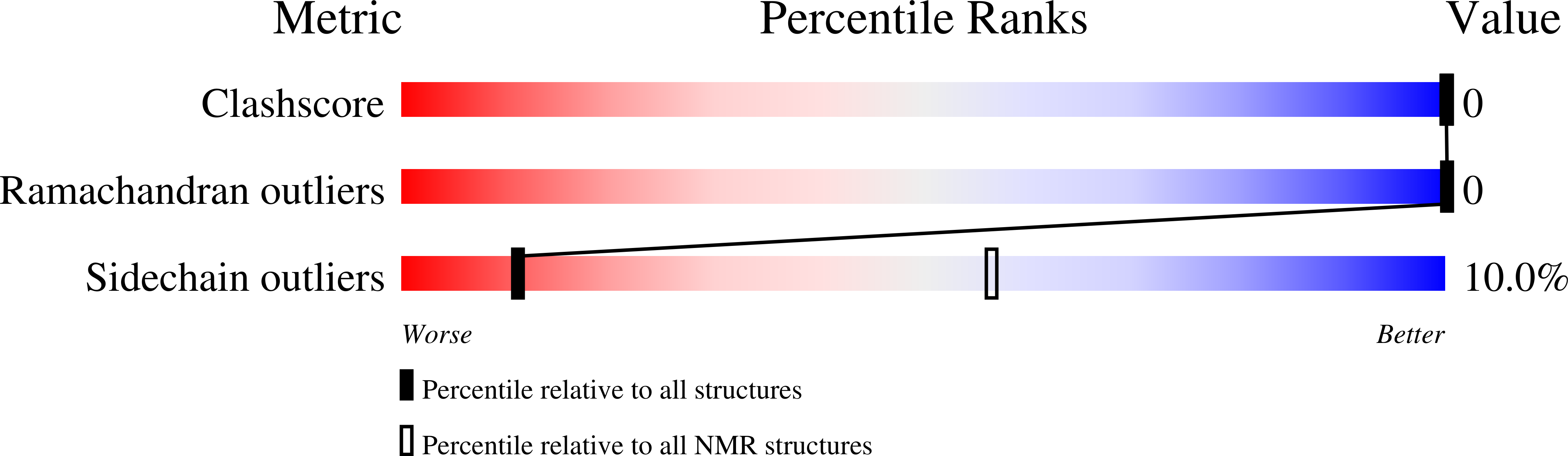Backbone and side-chain 1H, 13C and 15N assignments of the ubiquitin-associated domain of human X-linked inhibitor of apoptosis protein
Hui, S.K., Tse, M.K., Yang, Y., Wong, B.C., Sze, K.H.(2010) Biomol NMR Assign 4: 13-15
- PubMed: 19916060
- DOI: https://doi.org/10.1007/s12104-009-9197-x
- Primary Citation of Related Structures:
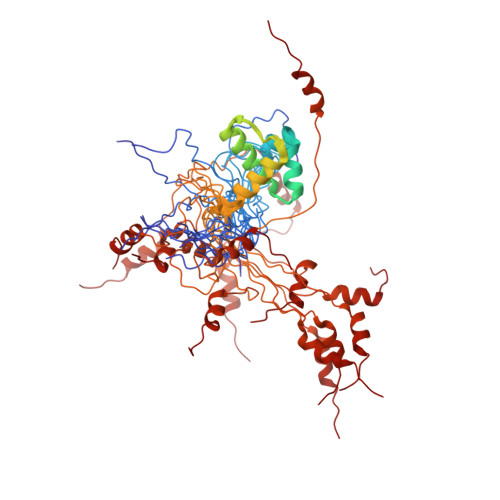
2KNA - PubMed Abstract:
X-linked inhibitor of apoptosis protein (XIAP), a leading member of the family of inhibitor of apoptosis (IAP) proteins, is considered as the most potent and versatile inhibitor of caspases and apoptosis. It has been reported that XIAP is frequently overexpressed in cancer and its expression level is implicated in contributing to tumorigenesis, disease progression, chemoresistance and poor patient-survival. Therefore, XIAP is one of the leading targets in drug development for cancer therapy. Recently, based on bioinformatics study, a previously unrecognized but evolutionarily conserved ubiquitin-associated (UBA) domain in IAPs was identified. The UBA domain is found to be essential for the oncogenic potential of IAP, to maintain endothelial cell survival and to protect cells from TNF-alpha-induced apoptosis. Moreover, the UBA domain is required for XIAP to activate NF-kappaB. In the present study, we report the near complete resonance assignments of the UBA domain-containing region of human XIAP protein. Secondary structure prediction based on chemical shift index (CSI) analysis reveals that the protein is predominately alpha-helical, which is consistent with the structures of known UBA proteins.
Organizational Affiliation:
Department of Chemistry and Open Laboratory of Chemical Biology of the Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong SAR, People's Republic of China.