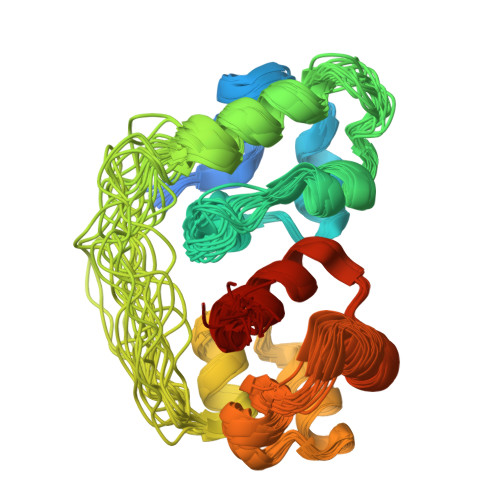
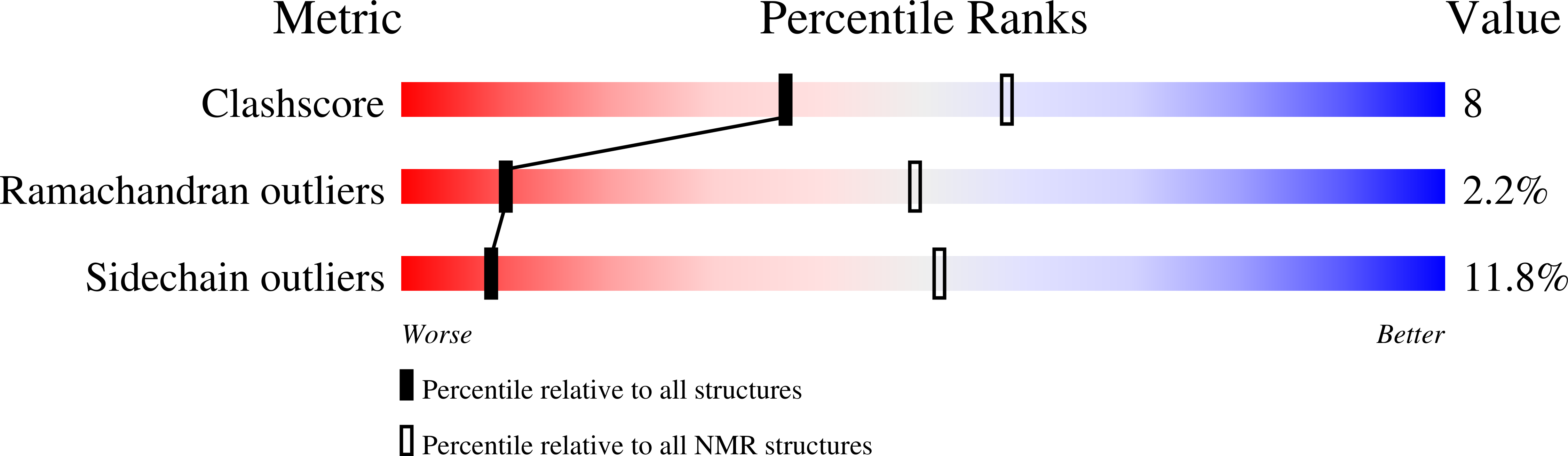A nuclear localization signal at the SAM-SAM domain interface of AIDA-1 suggests a requirement for domain uncoupling prior to nuclear import.
Kurabi, A., Brener, S., Mobli, M., Kwan, J.J., Donaldson, L.W.(2009) J Mol Biology 392: 1168-1177
- PubMed: 19666031
- DOI: https://doi.org/10.1016/j.jmb.2009.08.004
- Primary Citation of Related Structures:
2KIV - PubMed Abstract:
The neuronal scaffolding protein AIDA-1 is believed to act as a convener of signals arising at postsynaptic densities. Among the readily identifiable domains in AIDA-1, two closely juxtaposed sterile alpha motif (SAM) domains and a phosphotyrosine binding domain are located within the C-terminus of the longest splice variant and exclusively in four shorter splice variants. As a first step towards understanding the possible emergent properties arising from this assembly of ligand binding domains, we have used NMR methods to solve the first structure of a SAM domain tandem. Separated by a 15-aa linker, the two SAM domains are fused in a head-to-tail orientation that has been observed in other hetero- and homotypic SAM domain structures. The basic nuclear import signal for AIDA-1 is buried at the interface between the two SAM domains. An observed disparity between the thermal stabilities of the two SAM domains suggests a mechanism whereby the second SAM domain decouples from the first SAM domain to facilitate translocation of AIDA-1 to the nucleus.
Organizational Affiliation:
Department of Biology, York University, Toronto, Ontario, Canada.