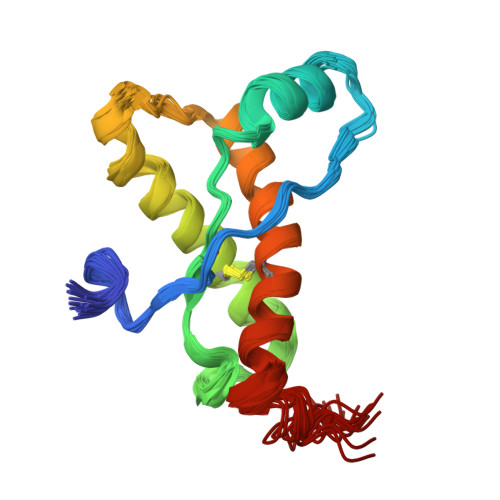
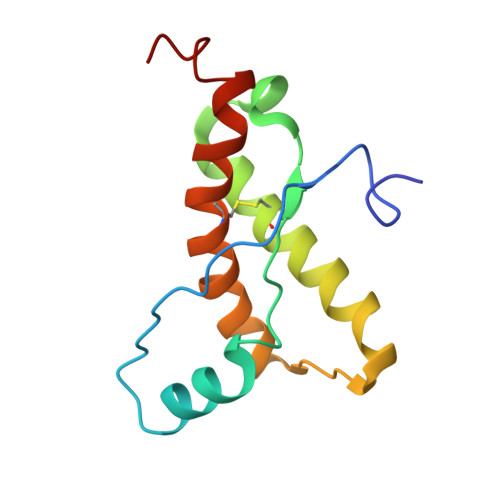
Prion Protein NMR Structure from Tammar Wallaby (Macropus eugenii) Shows that the beta2-alpha2 Loop Is Modulated by Long-Range Sequence Effects.
Christen, B., Hornemann, S., Damberger, F.F., Wuthrich, K.(2009) J Mol Biology 389: 833-845
- PubMed: 19393664
- DOI: https://doi.org/10.1016/j.jmb.2009.04.040
- Primary Citation of Related Structures:
2KFL, 2KFM, 2KFO - PubMed Abstract:
NMR structures are presented for the recombinant construct of residues 121-230 from the tammar wallaby (Macropus eugenii) prion protein (PrP) twPrP(121-230) and for the variant mouse PrPs mPrP[Y225A,Y226A](121-231) and mPrP[V166A](121-231) at 20 degrees C and pH 4.5. All three proteins exhibit the same global architecture as seen in other recombinant PrP(C)s (cellular isoforms of PrP) and shown to prevail in natural bovine PrP(C). Special interest was focused on a loop that connects the beta2-strand with helix alpha2 in the PrP(C) fold, since there are indications from in vivo experiments that this local structural feature affects the susceptibility of transgenic mice to transmissible spongiform encephalopathies. This beta2-alpha2 loop and helix alpha3 form a solvent-accessible contiguous epitope, which has been proposed to be the recognition area for a hypothetical chaperone, the "protein X". This hypothetical chaperone would affect the conversion of PrP(C) into the disease-related scrapie form (PrP(Sc)) by moderating intermolecular interactions related to the transmission barrier of transmissible spongiform encephalopathies between different species. In contrast to mPrP(121-231) and most other mammalian PrP(C)s, the beta2-alpha2 loop is well defined at 20 degrees C in tammar wallaby PrP and in the two aforementioned variants of mPrP, showing that long-range interactions with helix alpha3 can have an overriding influence on the structural definition of the beta2-alpha2 loop. Further NMR studies with two variant mPrPs, mPrP[Y225A](121-231) and mPrP[Y226A](121-231), showed that these interactions are dominantly mediated by close contacts between residues 166 and 225. The results of the present study then lead to the intriguing indication that well-defined long-range intramolecular interactions could act as regulators of the functional specificity of PrP(C).
Organizational Affiliation:
Institute of Molecular Biology and Biophysics, ETH Zurich, Switzerland.