Apo and Calcium-bound Crystal Structures of Alpha-11 Giardin, an Unusual Annexin from Giardia lamblia
Pathuri, P., Nguyen, E.T., Svard, S.G., Luecke, H.(2007) J Mol Biol 368: 493-508
- PubMed: 17355882
- DOI: https://doi.org/10.1016/j.jmb.2007.02.016
- Primary Citation of Related Structures:
2II2, 2IIC - PubMed Abstract:
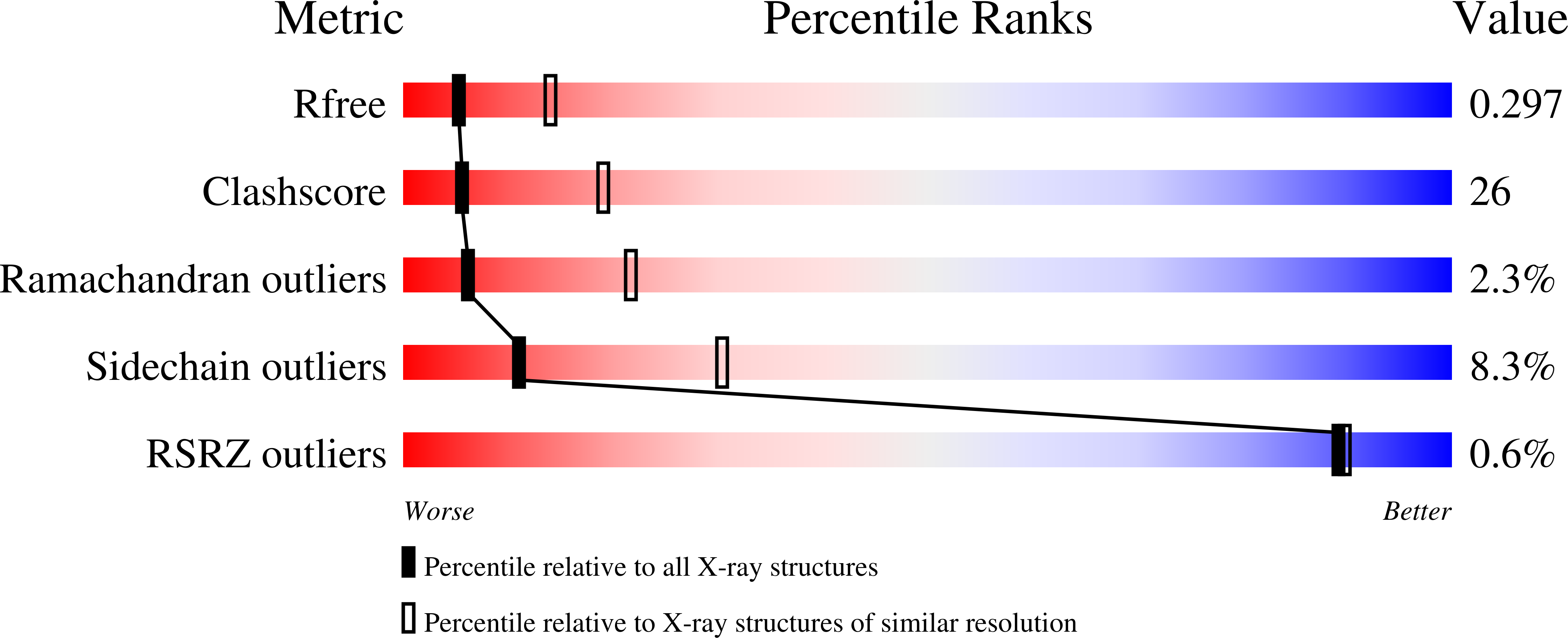
Alpha-11 giardin is a member of the multi-gene alpha-giardin family in the intestinal protozoan, Giardia lamblia. This gene family shares an ancestry with the annexin super family, whose common characteristic is calcium-dependent binding to membranes that contain acidic phospholipids. Several alpha giardins are highly expressed during parasite-induced diarrhea in humans. Despite being a member of a large family of proteins, little is known about the function and cellular localization of alpha-11 giardin, although giardins are often associated with the cytoskeleton. It has been shown that Giardia exhibits high levels of alpha-11 giardin mRNA transcript throughout its life cycle; however, constitutive over-expression of this protein is lethal to the parasite. Determining the three-dimensional structure of an alpha-giardin is essential to identifying functional domains shared in the alpha-giardin family. Here we report the crystal structures of the apo and Ca(2+)-bound forms of alpha-11 giardin, the first alpha giardin to be characterized structurally. Crystals of apo and Ca(2+)-bound alpha-11 giardin diffracted to 1.1 A and 2.93 A, respectively. The crystal structure of selenium-substituted apo alpha-11 giardin reveals a planar array of four tandem repeats of predominantly alpha-helical domains, reminiscent of previously determined annexin structures, making this the highest-resolution structure of an annexin to date. The apo alpha-11 giardin structure also reveals a hydrophobic core formed between repeats I/IV and II/III, a region typically hydrophilic in other annexins. Surprisingly, the Ca(2+)-bound structure contains only a single calcium ion, located in the DE loop of repeat I and coordinated differently from the two types of calcium sites observed in previous annexin structures. The apo and Ca(2+)-bound alpha-11 giardin structures assume overall similar conformations; however, Ca(2+)-bound alpha-11 giardin crystallized in a lower-symmetry space group with four molecules in the asymmetric unit. Vesicle-binding studies suggest that alpha-11 giardin, unlike most other annexins, does not bind to vesicles composed of acidic phospholipids in a calcium-dependent manner.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, University of California, Irvine, CA 92697, USA.